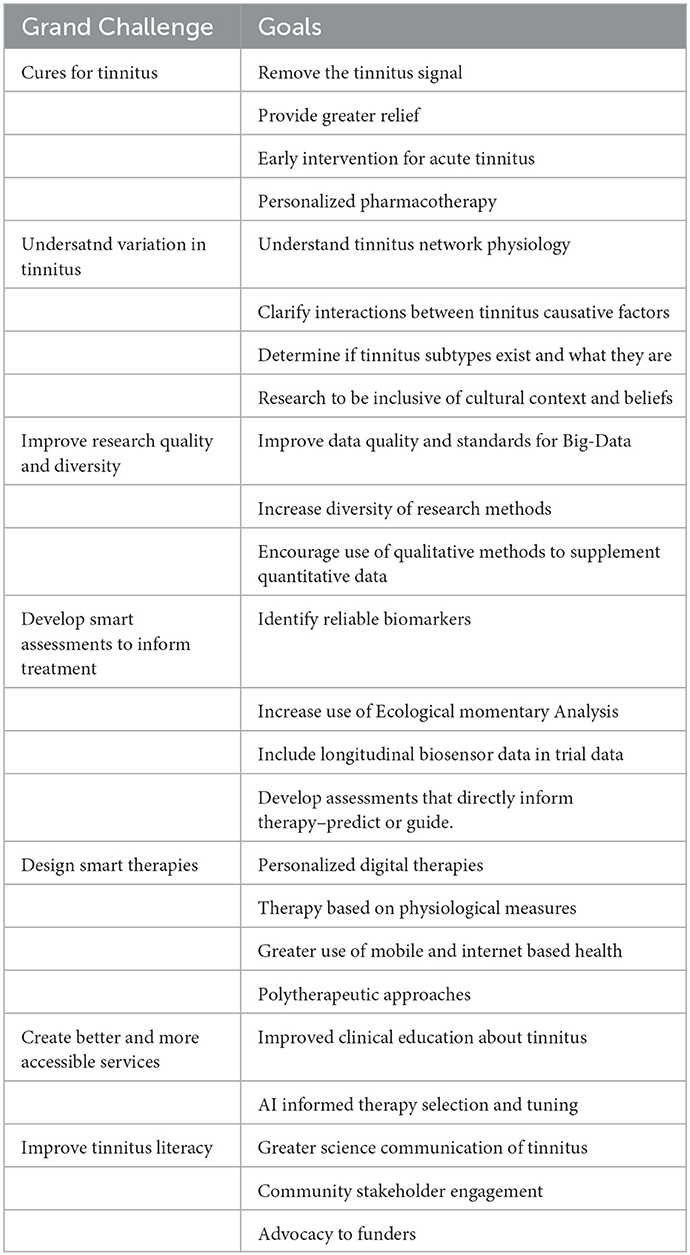
Specialty grand challenge: Smarter solutions for tinnitus
- Section of Audiology, The University of Auckland, Auckland, New Zealand
1. Introduction
This Specialty Grand Challenge is the authors perspective of critical needs in Otology and Audiology tinnitus practice and the innovative research that is needed to address them. Subjective tinnitus is the perception of sound in the absence of a physical sound. Is one of the most common complaints seen by Audiologists and Otologists. The aim of the Tinnitus specialty section of Frontiers in Audiology and Otology is to provide a rigorously peer-reviewed academic forum for the critical evaluation of current and future practice in tinnitus and the fundamental and clinical research required to address key challenges. The rapid growth in tinnitus publications in Otology and Audiology particularly in the last 10 years illustrates the need for a specialty section focused on tinnitus in our fields (Figure 1). This journal specialty section will become a key resource for clinicians and researchers looking for innovative approaches to address the complex problem of tinnitus. Addressing the research challenges outlined here are hypothesized to be essential for future clinical practice.
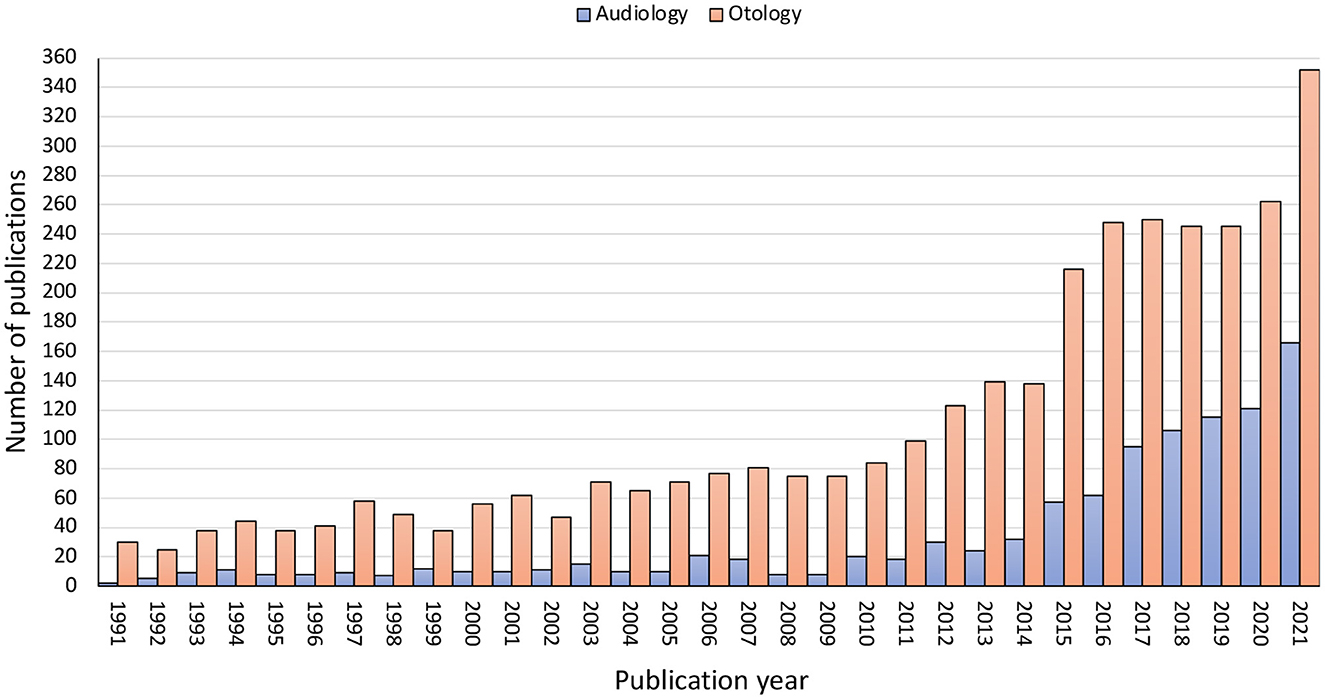
Figure 1. Research publications by year for the past 30 years for the PubMed search terms “Tinnitus and Audiology” and “Tinnitus and Otology”.
Tinnitus is a complex hybrid of perception (hearing a sound) and reaction (the extent to which it is a problem). To differentiate problem from non-problem tinnitus the term tinnitus disorder has recently be coined (De Ridder et al., 2021). Tinnitus is the conscious awareness of a tonal or composite noise for which there is no identifiable corresponding external acoustic source whereas Tinnitus Disorder occurs when tinnitus is associated with emotional distress, cognitive dysfunction, and/or autonomic arousal, leading to behavioral changes and functional disability. A clear dichotomy between tinnitus and tinnitus disorder doesn't present itself clinically; when does tinnitus become a slight-mild tinnitus disorder? However, the intent of the definition does illustrate the complexity of tinnitus and the challenge it presents to clinical practice and research. On one side of the coin tinnitus is the perception of sound other it is a disorder due to psychological responses. A satisfactory treatment for tinnitus will arise when we can either eliminate the sound, extinguish the disorder, or both.
2. Challenges
2.1. Find a cure for tinnitus
The biggest challenge in our field is removing the tinnitus sound. It is worth noting that the Collins Dictionary defines cure as when “medical treatments cure an illness or injury, they cause it to end or disappear” or “they make the person well” while the Mariam-Webster dictionary defines cure as “recovery or relief from a disease.” If a cure is to remove tinnitus we are a long way from achieving this goal, if it is relief we are much closer. Much of the commentary that follows should be considered in light of the aspirational goal of ending tinnitus (treatments to make it disappear) but also the pragmatic need to have more effective relieving therapies.
Current therapies for tinnitus are largely symptomatic; a problem shared with many neurological disorders (Boison, 2021). The failure to turn-off the tinnitus is at least in part due to reactive neural-plasticity following ear or head injury. As early as the 1940's it was hypothesized that if you could cure hearing loss you could cure tinnitus (Fowler, 1948). Hearing aids and cochlear implants provide some evidence that this could be the case, but would complete organic repair of hearing loss eliminate tinnitus? This might depend on if afferent cochlear-neural activity was also completely restored and if plasticity resulting from the injury could be fully reversed. Pharmacological treatments for tinnitus are more likely to suppress tinnitus activity than to remove it; as the igniting ear or head injury remains. The best possibility for tinnitus elimination may lay in the acute suppression of changed activity following injury, thereby reducing the possibility of chronification and centralizing of activity driving perception (Mulders and Robertson, 2011). This may include the treatment of acute neuroinflammation (Adcock and Vanneste, 2022). In the management of pain, there has been considerable progress in efforts to predict the risk and mitigate the transition from acute to chronic pain (Traeger et al., 2016; George et al., 2020). Socially and clinically a paradigm shift may be required that gives greater consideration to the acute presentation of tinnitus and its immediate treatment (Vielsmeier et al., 2020). The window of opportunity for a cure may be very short (Mulders and Robertson, 2011). A cure in such circumstances would require a substantial mind shift amongst the population experiencing what they may interpret as transient tinnitus, and professionals conservative management of watchful waiting. To address this grand challenge we need to increase use of physiological measurements alongside therapies to piece together tinnitus sequalae (Schmidt et al., 2017; Lan et al., 2021) and temporal details of therapy, we need to be more attentive to duration of tinnitus and understand psychosocial implications of, and education required for, acute tinnitus treatment. Developers of future tinnitus treatments should consider where an individual's tinnitus sits in the sequelae of tinnitus development (Simoes et al., 2021). The complex nature of tinnitus requires consideration of tinnitus network biology and potentially use of a poly-therapeutics approach targeting tinnitus-causing networks rather than a single mechanisms within a network (He et al., 2017). Personalized treatments that combine several therapeutic actions may become more common (Tzounopoulos et al., 2019; Searchfield et al., 2021a).
2.2. Understand variation in tinnitus
Effective cure(s) for all tinnitus, elimination, or relief, are going to require an accurate multifactorial understanding of individual differences. Over the last decade there has been increased recognition of tinnitus heterogeneity and the drives for personalized therapeutic approaches (Van de Heyning et al., 2015; Cederroth et al., 2019; McFerran et al., 2019; Simoes et al., 2019). No two patients are likely to experience the same tinnitus; their perception and rection will differ as will their personality (Searchfield, 2014), genetics (Maas et al., 2017; Veile et al., 2018) lifestyle (Veile et al., 2018) beliefs (Fludra et al., 2022) and whether they are seeking help (Rademaker et al., 2021). Behavioral neuroimaging and animal studies demonstrate tinnitus-associated changes in not only the auditory system but also other sensory systems and networks related to memory, emotion, attention, and stress (Knobel and Sanchez, 2008; Roberts et al., 2010; Henry et al., 2014; Simonetti and Oiticica, 2015; Leaver et al., 2016; Henton and Tzounopoulos, 2021). These broad functional neural regions are consistent with tinnitus impact. A research challenge is to advance individualized identification of tinnitus-related network activity and if tinnitus cannot be turned off at the source, we need to target treatment toward affected networks. To fully understand tinnitus, we must explore the interaction of physiology, genetics, psychology and culture. Such understandings with help address specific needs within different populations so we can reduce tinnitus' educational, social and vocational effects (Bhatt, 2018).
Parts of the tinnitus population may be more responsive to a therapy than others. It may be possible to subtype tinnitus based on its etiology (noise, ototoxicity, or blast trauma), symptoms (anxiety, depression, insomnia) stage of pathogenesis (acute, chronic), and severity of symptoms (non-bothersome, bothersome, disabling) (Simoes et al., 2019; Genitsaridi et al., 2020; Rademaker et al., 2021; Smith et al., 2021; Mohan et al., 2022). Physiological markers may differentiate tinnitus mechanisms differing between individuals. However, discrete subtypes may also not exist. For example, clustering according to the minimum masking level is weak and suggests a continuum rather than discrete subtypes (Santacruz et al., 2021). Also because tinnitus is not static but changes between, and during, days [possibly following circadian rhythms (Zacharia et al., 2014)] we should explore therapies tuned to the individual and attuned to possible temporal fluctuations. To address this variability, we need to be able to ascertain individual differences that predict the most appropriate therapy.
Tinnitus research has been dominated by western cultural perspectives of health. It is important that culture and beliefs are accounted for and that there is greater diversity in populations tested (Choi et al., 2020). Treatments may not work as equally well-across diverse populations groups due to genetic differences and/or cultural beliefs. Tinnitus research in low-socio economic regions needs to be supported, this not only benefits these populations it enriches all understanding of tinnitus.
2.3. Improve research quality and diversity
To tackle the wicked problem of tinnitus heterogeneity we must implement a range of research methods that are of high quality but also appropriate to the questions being asked and the stage of research. Data-driven tinnitus research requires either large datasets to account for heterogeneity or in-depth repeated measures in individuals. Artificial intelligence can be applied to these datasets such that it can extract patterns in big-data or it learns personal preferences for decision making in individuals (Song et al., 2017; Schlee et al., 2021; Searchfield et al., 2021b). Complex relationships between genetic, demographic, lifestyle, and other environmental factors create the variance in tinnitus datasets (Lopez-Escamez et al., 2016). Efforts toward big-data analaysis are hampered when the natural variation in tinnitus is accompanied by variation in assessment measures. There have been efforts to develop and adopt a core set of measures to be obtained on a regular basis in human participant research (Hall et al., 2018b). This standardized data can then be pooled to extract patterns (Schlee et al., 2018). A variety of data is important because of the absence of a gold-standard objective measure of tinnitus. Measures of hearing and tinnitus characteristics (pitch, loudness and masking) may aid understanding of individual differences, even if at a group level they have limited prognostic value. There are several widely used tinnitus questionnaires developed using modern understand of concepts of responsiveness to treatment, e.g., The Tinnitus Functional Index (Meikle et al., 2012) and Tinnitus Primary Function Index (Tyler et al., 2014). These have been tested in several countries and understanding of degree of change corresponding to meaningful improvement is becoming clear. The creation of new questionnaires must be considered against the possibility that they will simply further dilute the uniformity of data. On the other hand, many older questionnaires may have limitations and should be retired from use, or at least accompanied by a modern alternative. The diagnosis of comorbidities such as depression, anxiety and cognitive impairment are also important for large datasets. There needs to be some flexibility in what is included overtime in these datasets as new evidence suggests more sensitive measures or previously undocumented predictive factors.
The quality of Tinnitus research appears to be improving, recently several reasonably powered well-controlled trials have been published testing therapies (Scherer et al., 2019; Conlon et al., 2020; Hall et al., 2022; Searchfield and Sanders, 2022). These studies clearly state objectives, methods and have a control to provide a comparison of the effect. Participant selection and allocation to groups minimizes bias and enables equivalency of the groups. Such randomized controlled trials do have shortcomings: they take a great deal of time, are expensive to run with strict adherence protocols (Pham et al., 2016). In areas of rapid technology development flexibility to technology change can be accommodated using “evaluate intervention” principles (Mohr et al., 2015). Because RCTs are carefully managed sometimes the results may not reflect real-world efficacy or useability. Other times the question being explored is at an early stage of research. In such cases smaller proof of concept, feasibility and pilot trials are needed. These types of research can all be of high quality, but the conclusions need to tempered relative to the design. Claims of effectiveness in small samples must relate to the sample tested and should not be generalized to wider groups. Case-reports and single case designs can be very valuable for assessing causal relationships between interventions and outcomes (Lobo et al., 2017). This methodology relies on examination of within individual differences over time and intervention. Instead of measurements across many people the dependent variable is measured many times within individual's with varying phases (Lobo et al., 2017).
Most tinnitus research follows the quantitative tradition. Gaps in our understanding of tinnitus, particularly from the tinnitus suffers perspective could be filled by more extensive use of qualitative research (Heinrich et al., 2016; Durai and Searchfield, 2017; Pryce et al., 2018; Taylor et al., 2020). All these research methods add depth to our tinnitus understanding. This specialty section encourages submissions of quality research employing a range of research approaches.
2.4. Develop smart assessments to inform treatment
The absence of an objective measure of tinnitus has been a longstanding excuse for the failure to find a tinnitus cure. The absence of a gold-standard objective measure is understandable when the complexity and hetergeniety of tinnitus is considered. Genetic and blood-based biomarkers for tinnitus are emerging (Szczepek et al., 2014; Haider et al., 2020) and we are getting closer to being able to differentiate between tinnitus and non-tinnitus activity by examining imaging and EEG activity (Vanneste et al., 2018; Liu et al., 2019). Metabolic, hemostatic, inflammatory, endocrine, immunological, oxidative and neurologic markers may all contribute to tinnitus diagnosis and categorization (Kang et al., 2021). With the aid of machine learning algorithms we may soon be able to accurately distinguish tinnitus from a non-tinnitus neural activity at an individual level that can help inform treatment (Han et al., 2019; Durai et al., 2020). A very promising avenue for tinnitus assessment is ecological momentary analysis using questionnaires (Deutsch and Piccirillo, 2021) or behavioral measures coupled with wearable sensors measuring physiological function (Searchfield et al., 2021b). Ecological Momentary Analysis samples subjective states regularly while biosensors for example within smartwatches can objectively monitor physiology (Schlee et al., 2016; Goldberg et al., 2017). For sleep Actigraphy is an inexpensive method for recording and then estimating sleep activity (wakefulness and sleep from timing, intensity, and duration of movements) using inertial sensors available in smart watches (De Zambotti et al., 2019). Real-time monitoring of tinnitus symptoms alongside physiology provides longitudinal information that may aid diagnosis and inform treatment, including medication dosage. Similarly, the measures may inform the selection and/or engagement with pharmacotherapy, sound therapy or counseling, thus enabling smart therapies.
2.5. Design smarter therapies
Fowler (1948) believed that a cure for hearing loss would cure tinnitus. We are someway off restoring normal hearing, but hearing aids and, to a certain degree, cochlear implants, have become regular tools in tinnitus management (Shekhawat et al., 2013). Hearing Aids are used as tinnitus management tools to reduce accompanying hearing handicap, reduce the levels of attention paid to tinnitus, compensate for deafferentation, and by raising the audibility of environmental sounds mask tinnitus (Shekhawat et al., 2013). Many hearing aids feature tinnitus settings with variations of sound for masking or Tinnitus Retraining therapy (Hoare et al., 2014). Further research is still needed to understand the mechanisms of effect and optimisation of amplification and sounds for tinnitus therapy. A particular challenge is to adapt these to individual characteristics and needs (Searchfield et al., 2017). The release of Hearables (earbuds with hearing aid features) by large consumer electronics companies presents further avenues for pathway for tinnitus technology innovation, particularly due to the potential to incorporate biosensors within these devices (Searchfield et al., 2021b). The ear may replace the wrist as the preferred region to record biological signals from. In the future bio-signals may inform selection or dosage of sound-based therapies.
Digital health has enabled growth in various tinnitus analogs of face-face therapies as well as new treatment approaches. The internet-based Cognitive Behavioral Thearpy (iCBT) approach has been most widely researched. ICBT consists of education and information about tinnitus, management strategies, training for self-directed psychological approaches, social support, and monitoring of tinnitus (Mehta et al., 2019). Many self-help tinnitus lifestyle apps are available online, but very few apps have been scientifically validated or tested for efficacy. Hearing aid manufacturers have developed companion apps for their aids to offer additional tinnitus functions and these have also been trialed with cochlear implants. There are also large gaps in our knowledge about the effectiveness and end-user engagement with these apps. Digital therapeutics, evidenced-based software prescribed similar to drugs are becoming more widespread. Digital therapeutics are available for variations conditions including mental health (Hong et al., 2021). In the case of tinnitus the strategies prescribed may include, information, counseling, sound therapy and auditory training (Searchfield and Sanders, 2022). These may be a form of network therapy, if this is the case physiological measures should be able to demonstrate changes in connectivity between and within networks. Digital therapeutics may employ “Serious games” to maintain motivation and compliance with auditory training and may modify different psychoacoustic or cognitive processes (Wise et al., 2016). In a basic form the goal is to train the person with tinnitus to focus on target sounds while suppressing background sounds (Searchfield et al., 2007). More widespread work needs to be done to explore how these technologies can be used in an effective manner for tinnitus. These digital therapeutics could also be paired with medications to potentiate each therapy's action (Searchfield et al., 2020). Bimodal stimulation has become a popular avenue to exploit neuroplasticity by pairing sound with vagus nerve or trigeminal nerve stimulation (Riffle et al., 2021). These approaches offer an exciting window into how multiple actions can be coupled to improve the degree and speed of tinnitus therapies.
Currently the selection and implementation of therapy is based largely on expert opinion. We need to become smarter in selecting between therapies and potentially tuning them to individual needs and physiological state. Tailored diagnosis and treatment of tinnitus may be possible (Tzounopoulos et al., 2019). AI-driven decision-making and adjustment may enable smart therapy. Machine learning algorithms may use personality (Durai et al., 2017; Kleinstäuber et al., 2018), tinnitus severity (Mazurek et al., 2006), tinnitus location (Theodoroff et al., 2014) gender (Vanneste et al., 2012; Lugo et al., 2019; Niemann et al., 2020; Van der Wal et al., 2020) and physiological measures such as Magnetic Resonance Imaging and Electroencephalography (Han et al., 2019; Durai et al., 2020; Allgaier et al., 2021) to predict or modify therapies. In the future it may be possible to use sensors (e.g., Global Positioning Satellite locators, microphones, gyroscopes, accelerometers, Electrocardiogram and Galvanic Skin Response measures) in smartphones or peripheral wearable devices such as smart-watches and hearables to aid therapy selection and dosing. How to best to incorporate AI and biosensors in tinnitus research and practice is at present an open question (Searchfield et al., 2021b).
2.6. Create better and more accessible services
Many different healthcare professions play a role in the holistic management of tinnitus, including psychiatrists, psychologists and psychical therapists. The focus of this Journal is Audiology and Otology. Otologists play a critical role the identification and treatment of underlying casual mechanisms of tinnitus. Although a treatable cause of tinnitus is absent in most cases of tinnitus if it is then treatment may resolve or improve the tinnitus. The otologist also plays an important role in the identification and management of co-morbid conditions, including anxiety and depression (Shi et al., 2014). Following medical evaluation and treatment, audiologists often provide a combination of sound therapy, with hearing aids, and counseling. This therapy is effective for many but is of limited or no benefit for others (Brennan-Jones et al., 2020). Despite being within scope of practice relatively few audiologists provide comprehensive tinnitus services. There appears to be several factors contributing to this reluctance among audiologists, including the absence in some countries of third party coverage for tinnitus management costs, the complexity and ambiguity of tinnitus evaluations, and self-perceived inadequate skills in counseling (Henry et al., 2019). There is a need for greater clinical education in tinnitus and effective tinnitus practice tools that ease clinician burden and support effective practice. Practice guidelines exist for the USA and Europe that reflect the professional skills of the primary professionals involved in these regions (Tunkel et al., 2014; Cima et al., 2019). However these are fairly generic and in some cases recommendations are not definitive due to limited evidence. Decision support systems created from Big Data may guide effective treatment selection to aid clinicians (Sarafidis et al., 2021). Better service structures, support and tools for tinnitus care may encourage more clinicians to take up tinnitus practice.
The scarcity of healthcare practioners specializing in tinnitus has accelerated the development of online and mobile therapies. The merits of self-help, self-directed with some clinician involvement, and traditional clinic based services should be explored. Provided that communication technology is available and the cost of service is suitable Telehealth should enable widespread access to services. It is important that research is undertaken to investigate tinnitus telehealth and that the international provision of services is culturally appropriate for the user of such services.
2.7. Greater tinnitus literacy
Tinnitus appears to be often misunderstood by the public and some health professionals. There is a general inadequacy in training for audiologists and the unstandardized content of education programs has been highlighted (Henry et al., 2019). This may be the case for other professions. There is a need to identify core competencies to be taught across counseling, and use of sound therapy. There also needs to be sufficient research literacy amongst audiologists and otologists to adapt core skills to new therapies. The concept of evidence-based or evidence-informed practice is no longer a new idea. But there still appears some willingness for clinicians to recommend interventions with limited evidence base (Henry et al., 2019).
Effective translation of research requires broad participation of stakeholders including services, clinicians, patients, and their communities. Historically the end users of treatments have had limited input, or have been left out of discussions of research needs. Efforts have been made internationally to ensure the patient voice is included in discussions including being involved in co-design (Hall et al., 2018a,b; Watts et al., 2018). There is a need for researchers to listen to and act on patients desires for cures and reciprocity from patients to respect research, and researchers, that focus on symptomatic treatment.
Clinicians and researchers need to ensure that science communication is effective and that tools developed for tinnitus assessment and management are linguistically and culturally appropriate (Beukes et al., 2020). Compared to some common health problems funding for tinnitus research is low and in some countries tinnitus treatment is not reimbursed by insurers or funded publicly. There are currently only a few non-government organizations that solely fund tinnitus research (e.g., the American and British Tinnitus Associations) so researchers must seek funding in competition with other health problems. To address this patient groups, clinicians and researchers need to work together as joint stakeholders and advocate to governments and non-government organizations to place a higher priority on funding tinnitus.
3. Conclusion
Tinnitus is a complex problem regularly encountered by otologists and audiologists. The personal perspectives of Tinnitus Grand Challenges presented in this article are open to debate (Table 1). The mission of the Tinnitus specialty section of Frontiers in Audiology and Otology is to publish rigorously peer-reviewed research at the forefront of tinnitus theory, assessment, and management, with a focus on translation and application to Audiology and Otology. The section is open to all methodological approaches including qualitative, quantitative, and mixed methods. The section is a forum for researchers in Audiology, Otology, and related sciences to demonstrate research and clinical progress. The ultimate goal for tinnitus research should be to find cures, but in striving toward this aspirational goal, research will result in more effective assessment, management and service delivery. We need to carry stakeholders with us in this journey through compelling science communication. Tinnitus research is thriving despite its complexity and funding constraints. Through advocacy we as a community can raise tinnitus as a serious issue deserving of more funding for research and improved clinical services.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
GS's tinnitus research is currently funded by Te Titoki Mataora, the MedTech Research Translator, New Zealand.
Conflict of interest
GS was a founder of and has financial interest in TinnitusTunes and TrueSilence Therapeutics Inc., tinnitus treatment companies.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adcock, K., Vanneste, S. (2022). Neuroinflammation in tinnitus. Curr. Otorhinol. Rep. 10, 322–328. doi: 10.1007/s40136-022-00411-8
Allgaier, J., Neff, P., Schlee, W., Schoisswohl, S., Pryss, R. (2021). Deep learning end-to-end approach for the prediction of tinnitus based on EEG Data. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 816–819. doi: 10.1109/EMBC46164.2021.9629964
Beukes, E. W., Fagelson, M., Aronson, E. P., Munoz, M. F., Andersson, G., Manchaiah, V. (2020). Readability following cultural and linguistic adaptations of an internet-based intervention for tinnitus for use in the United States. Am. J. Audiol. 29, 97–109. doi: 10.1044/2019_AJA-19-00014
Bhatt, I. S. (2018). Prevalence of and risk factors for tinnitus and tinnitus-related handicap in a college-aged population. Ear Hear 39, 517–526. doi: 10.1097/AUD.0000000000000503
Boison, D. (2021). Specialty grand challenge for brain disease mechanisms. Front. Mol. Neurosci. 14, 689903. doi: 10.3389/fnmol.2021.689903
Brennan-Jones, C. G., Thomas, A., Hoare, D. J., Sereda, M. (2020). Cochrane corner: sound therapy (using amplification devices and/or sound generators) for tinnitus. Int. J. Audiol. 59, 161–165. doi: 10.1080/14992027.2019.1643503
Cederroth, C. R., Gallus, S., Hall, D. A., Kleinjung, T., Langguth, B., Maruotti, A., et al. (2019). Towards an understanding of tinnitus heterogeneity. Front. Aging Neurosci. 11, 53. doi: 10.3389/fnagi.2019.00053
Choi, J. S., Yu, A. J., Voelker, C. C. J., Doherty, J. K., Oghalai, J. S., Fisher, L. M. (2020). Prevalence of tinnitus and associated factors among asian americans: results from a national sample. Laryngoscope 130, E933–E940. doi: 10.1002/lary.28535
Cima, R. F. F., Mazurek, B., Haider, H., Kikidis, D., Lapira, A., Norena, A., et al. (2019). A multidisciplinary European guideline for tinnitus: diagnostics, assessment, and treatment. HNO 67, 10–42. doi: 10.1007/s00106-019-0633-7
Conlon, B., Langguth, B., Hamilton, C., Hughes, S., Meade, E., Connor, C. O., et al. (2020). Bimodal neuromodulation combining sound and tongue stimulation reduces tinnitus symptoms in a large randomized clinical study. Sci. Trans. Med. 12, eabb2830. doi: 10.1126/scitranslmed.abb2830
De Ridder, D., Schlee, W., Vanneste, S., Londero, A., Weisz, N., Kleinjung, T., et al. (2021). Tinnitus and tinnitus disorder: theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 260, 1–25. doi: 10.1016/bs.pbr.2020.12.002
De Zambotti, M., Cellini, N., Goldstone, A., Colrain, I. M., Baker, F. C. (2019). Wearable sleep technology in clinical and research settings. Med. Sci. Sports Exerc. 51, 1538. doi: 10.1249/MSS.0000000000001947
Deutsch, B. C., Piccirillo, J. F. (2021). Momentary analysis of tinnitus: considering the patient. Curr. Top. Behav. Neurosci. 51, 383–401. doi: 10.1007/7854_2020_176
Durai, M., O'Keeffe, M. G., Searchfield, G. D. (2017). The personality profile of tinnitus sufferers and a nontinnitus control group. J. Am. Acad. Audiol. 28, 271–282. doi: 10.3766/jaaa.15103
Durai, M., Sanders, P., Doborjeh, Z., Doborjeh, M., Kasabov, N., Searchfield, G. (2020). Prediction of tinnitus masking benefit within a case series using a spiking neural network model. Prog. Brain Res. 260, 129–165. doi: 10.1016/bs.pbr.2020.08.003
Durai, M., Searchfield, G. D. (2017). A mixed-methods trial of broad band noise and nature sounds for tinnitus therapy: group and individual responses modeled under the adaptation level theory of tinnitus. Front. Aging Neurosci. 9, 44. doi: 10.3389/fnagi.2017.00044
Fludra, M., Gos, E., Kobosko, J., Karendys-Luszcz, K., Skarzynski, H. (2022). The role of religiosity and spirituality in helping polish subjects adapt to their tinnitus. J. Relig. Health. 1–18. doi: 10.1007/s10943-022-01527-3
Fowler, E. P. (1948). Nonvibratory tinnitus; factors underlying subaudible and audible irritations. Arch. Otolaryngol. 47, 29–36.
Genitsaridi, E., Hoare, D. J., Kypraios, T., Hall, D. A. (2020). A review and a framework of variables for defining and characterizing tinnitus subphenotypes. Brain Sci. 10, 938. doi: 10.3390/brainsci10120938
George, S. Z., Lentz, T. A., Beneciuk, J. M., Bhavsar, N. A., Mundt, J. M., Boissoneault, J. (2020). Framework for improving outcome prediction for acute to chronic low back pain transitions. Pain Rep. 5, e809. doi: 10.1097/PR9.0000000000000809
Goldberg, R. L., Piccirillo, M. L., Nicklaus, J., Skillington, A., Lenze, E., Rodebaugh, T. L., et al. (2017). Evaluation of ecological momentary assessment for tinnitus severity. JAMA Otolaryngol. Head Neck Surg. 143, 700–706. doi: 10.1001/jamaoto.2017.0020
Haider, H. F., Ribeiro, S. F., Martins, C., Ribeiro, D., Trigueiros, N., Szczepek, A. J., et al. (2020). Tinnitus, hearing loss and inflammatory processes in an older Portuguese population. Int. J. Audiol. 59, 323–332. doi: 10.1080/14992027.2019.1698775
Hall, D. A., Fackrell, K., Li, A. B., Thavayogan, R., Smith, S., Kennedy, V., et al. (2018a). A narrative synthesis of research evidence for tinnitus-related complaints as reported by patients and their significant others. Health Qual. Life Outcomes 16, 61. doi: 10.1186/s12955-018-0888-9
Hall, D. A., Pierzycki, R. H., Thomas, H., Greenberg, D., Sereda, M., Hoare, D. J. (2022). Systematic evaluation of the T30 neurostimulator treatment for tinnitus: a double-blind randomised placebo-controlled trial with open-label extension. Brain Sci. 12, 317. doi: 10.3390/brainsci12030317
Hall, D. A., Smith, H., Hibbert, A., Colley, V., Haider, H. F., Horobin, A., et al. (2018b). The COMiT'ID study: developing core outcome domains sets for clinical trials of sound-, psychology-, and pharmacology-based interventions for chronic subjective tinnitus in adults. Trends Hear. 22. doi: 10.1177/2331216518814384
Han, L., Na, Z., Chunli, L., Yuchen, C., Pengfei, Z., Hao, W., et al. (2019). Baseline functional connectivity features of neural network nodes can predict improvement after sound therapy through adjusted narrow band noise in tinnitus patients. Front. Neurosci. 13, 614. doi: 10.3389/fnins.2019.00614
He, J., Zhu, Y., Aa, J., Smith, P. F., De Ridder, D., Wang, G., et al. (2017). Brain metabolic changes in rats following acoustic trauma. Front. Neurosci. 11, 148. doi: 10.3389/fnins.2017.00148
Heinrich, S., Rozental, A., Carlbring, P., Andersson, G., Cotter, K., Weise, C. (2016). Treating tinnitus distress via the internet: a mixed methods approach of what makes patients seek help and stay motivated during internet-based cognitive behavior therapy. Int. Intervent. 4, 120–130. doi: 10.1016/j.invent.2016.04.001
Henry, J. A., Piskosz, M., Norena, A., Fournier, P. (2019). Audiologists and Tinnitus. Am J Audiol 28, 1059–1064. doi: 10.1044/2019_AJA-19-0070
Henry, J. A., Roberts, L. E., Caspary, D. M., Theodoroff, S. M., Salvi, R. J. (2014). Underlying mechanisms of tinnitus: review and clinical implications. J. Am. Acad. Audiol. 25, 5–22; quiz 126. doi: 10.3766/jaaa.25.1.2
Henton, A., Tzounopoulos, T. (2021). What's the buzz? The neuroscience and the treatment of tinnitus. Physiol. Rev. 101, 1609–1632. doi: 10.1152/physrev.00029.2020
Hoare, D. J., Searchfield, G. D., El Refaie, A., Henry, J. A. (2014). Sound therapy for tinnitus management: practicable options. J. Am. Acad. Audiol. 25, 62–75. doi: 10.3766/jaaa.25.1.5
Hong, J. S., Wasden, C., Han, D. H. (2021). Introduction of digital therapeutics. Comput. Methods Programs Biomed. 209, 106319. doi: 10.1016/j.cmpb.2021.106319
Kang, D. W., Kim, S. S., Park, D. C., Kim, S. H., Yeo, S. G. (2021). Objective and measurable biomarkers in chronic subjective tinnitus. Int. J. Mol. Sci. 22, 6619. doi: 10.3390/ijms22126619
Kleinstäuber, M., Weise, C., Andersson, G., Probst, T. (2018). Personality traits predict and moderate the outcome of internet-based cognitive behavioural therapy for chronic tinnitus. Int. J. Audiol. 57, 538–544. doi: 10.1080/14992027.2018.1432902
Knobel, K. A., Sanchez, T. G. (2008). Influence of silence and attention on tinnitus perception [comparative study randomized controlled trial research support, non-U.S. Gov't]. Otolaryngol. Head Neck Surg. 138, 18–22. doi: 10.1016/j.otohns.2007.09.023
Lan, L., Li, J., Chen, Y., Chen, W., Li, W., Zhao, F., et al. (2021). Alterations of brain activity and functional connectivity in transition from acute to chronic tinnitus. Hum. Brain Mapp. 42, 485–494. doi: 10.1002/hbm.25238
Leaver, A. M., Seydell-Greenwald, A., Rauschecker, J. P. (2016). Auditory-limbic interactions in chronic tinnitus: challenges for neuroimaging research. Hear Res. 334, 49–57. doi: 10.1016/j.heares.2015.08.005
Liu, Y., Niu, H., Zhu, J., Zhao, P., Yin, H., Ding, H., et al. (2019). Morphological neuroimaging biomarkers for tinnitus: evidence obtained by applying machine learning. Neural. Plasticity 2019, 1712342. doi: 10.1155/2019/1712342
Lobo, M. A., Moeyaert, M., Cunha, A. B., Babik, I. (2017). Single-case design, analysis, and quality assessment for intervention research. J. Neurol. Physical Therapy 41, 187. doi: 10.1097/NPT.0000000000000187
Lopez-Escamez, J. A., Bibas, T., Cima, R. F., Van de Heyning, P., Knipper, M., Mazurek, B., et al. (2016). Genetics of tinnitus: an emerging area for molecular diagnosis and drug development. Front Neurosci. 10, 377. doi: 10.3389/fnins.2016.00377
Lugo, A., Trpchevska, N., Liu, X., Biswas, R., Magnusson, C., Gallus, S., et al. (2019). Sex-specific association of tinnitus with suicide attempts. JAMA Otolaryngol. Head Neck Surg. 145, 685–687. doi: 10.1001/jamaoto.2019.0566
Maas, I. L., Brüggemann, P., Requena, T., Bulla, J., Edvall, N. K., Hjelmborg, J. V. B., et al. (2017). Genetic susceptibility to bilateral tinnitus in a Swedish twin cohort. Genet. Med. 19, 1007–1012. doi: 10.1038/gim.2017.4
Mazurek, B., Fischer, F., Haupt, H., Georgiewa, P., Reisshauer, A., Klapp, B. F. (2006). A modified version of tinnitus retraining therapy: observing long-term outcome and predictors. Audiol. Neurotol. 11, 276–286. doi: 10.1159/000093526
McFerran, D. J., Stockdale, D., Holme, R., Large, C. H., Baguley, D. M. (2019). Why is there no cure for tinnitus? Front. Neurosci. 13, 802. doi: 10.3389/fnins.2019.00802
Mehta, S., Peynenburg, V. A., Hadjistavropoulos, H. D. (2019). Internet-delivered cognitive behaviour therapy for chronic health conditions: a systematic review and meta-analysis. J. Behav. Med. 42, 169–187. doi: 10.1007/s10865-018-9984-x
Meikle, M. B., Henry, J. A., Griest, S. E., Stewart, B. J., Abrams, H. B., McArdle, R., et al. (2012). The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hearing 33, 53–176. doi: 10.1097/AUD.0b013e31822f67c0
Mohan, A., Leong, S. L., De Ridder, D., Vanneste, S. (2022). Symptom dimensions to address heterogeneity in tinnitus. Neurosci. Biobehav. Rev. 134, 104542. doi: 10.1016/j.neubiorev.2022.104542
Mohr, D. C., Schueller, S. M., Riley, W. T., Brown, C. H., Cuijpers, P., Duan, N., et al. (2015). Trials of intervention principles: evaluation methods for evolving behavioral intervention technologies. J. Med. Internet Res. 17, e166. doi: 10.2196/jmir.4391
Mulders, W. H., Robertson, D. (2011). Progressive centralization of midbrain hyperactivity after acoustic trauma. Neuroscience 192, 753–760. doi: 10.1016/j.neuroscience.2011.06.046
Niemann, U., Boecking, B., Brueggemann, P., Mazurek, B., Spiliopoulou, M. (2020). Gender-specific differences in patients with chronic tinnitus—baseline characteristics and treatment effects. Front. Neurosci. 14, 487. doi: 10.3389/fnins.2020.00487
Pham, Q., Wiljer, D., Cafazzo, J. A. (2016). Beyond the randomized controlled trial: a review of alternatives in mHealth clinical trial methods. JMIR mHealth and uHealth 4, e107. doi: 10.2196/mhealth.5720
Pryce, H., Durand, M.-A., Hall, A., Shaw, R., Culhane, B.-A., Swift, S., et al. (2018). The development of a decision aid for tinnitus. Int. J. Audiol. 57, 714–719. doi: 10.1080/14992027.2018.1468093
Rademaker, M. M., Stegeman, I., Brabers, A. E. M., de Jong, J. D., Stokroos, R. J., Smit, A. L. (2021). Differences in characteristics between people with tinnitus that seek help and that do not. Sci. Rep. 11, 22949. doi: 10.1038/s41598-021-01632-5
Riffle, T. L., Martel, D. T., Jones, G. R., Shore, S. E. (2021). Bimodal auditory electrical stimulation for the treatment of tinnitus: preclinical and clinical studies. Curr. Top Behav. Neurosci. 51, 295–323. doi: 10.1007/7854_2020_180
Roberts, L. E., Eggermont, J. J., Caspary, D. M., Shore, S. E., Melcher, J. R., Kaltenbach, J. A. (2010). Ringing ears: the neuroscience of tinnitus. J. Neurosci. 30, 14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010
Santacruz, J. L., de Kleine, E., van Dijk, P. (2021). Investigating the relation between minimum masking levels and hearing thresholds for tinnitus subtyping. Prog. Brain Res. 263, 81–94. doi: 10.1016/bs.pbr.2021.04.011
Sarafidis, M., Manta, O., Kouris, I., Schlee, W., Kikidis, D., Vellidou, E., et al. (2021). Why a clinical decision support system is needed for tinnitus? Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2075–2078. doi: 10.1109/EMBC46164.2021.9630137
Scherer, R. W., Formby, C., Group TRTTR. (2019). Effect of tinnitus retraining therapy vs standard of care on tinnitus-related quality of life: a randomized clinical trial. JAMA Otolaryngol. Head Neck Surg. 145, 597–608. doi: 10.1001/jamaoto.2019.0821
Schlee, W., Hall, D. A., Canlon, B., Cima, R. F., de Kleine, E., Hauck, F., et al. (2018). Innovations in doctoral training and research on tinnitus: the European School on interdisciplinary tinnitus research (ESIT) perspective. Front. Aging Neurosci. 9, 447. doi: 10.3389/fnagi.2017.00447
Schlee, W., Langguth, B., Pryss, R., Allgaier, J., Mulansky, L., Vogel, C., et al. (2021). Using big data to develop a clinical decision support system for tinnitus treatment. Curr. Top. Behav. Neurosci. 51, 175–189. doi: 10.1007/7854_2021_229
Schlee, W., Pryss, R. C., Probst, T., Schobel, J., Bachmeier, A., Reichert, M., et al. (2016). Measuring the moment-to-moment variability of tinnitus: the TrackYourTinnitus smart phone app. Front. Aging Neurosci. 8, 294. doi: 10.3389/fnagi.2016.00294
Schmidt, S. A., Carpenter-Thompson, J., Husain, F. T. (2017). Connectivity of precuneus to the default mode and dorsal attention networks: a possible invariant marker of long-term tinnitus. NeuroImage Clin. 16, 196–204. doi: 10.1016/j.nicl.2017.07.015
Searchfield, G., Spiegel, D., Poppe, T., Durai, M., Jensen, M., Kobayashi, K., et al. (2020). A proof-of-concept study comparing tinnitus and neural connectivity changes following multisensory perceptual training with and without a low-dose of fluoxetine. Int. J. Neurosci. 131, 433–444. doi: 10.1080/00207454.2020.1746310
Searchfield, G. D. (2014). Tinnitus what and where: an ecological framework [review]. Front. Neurol. 5, 271. doi: 10.3389/fneur.2014.00271
Searchfield, G. D., Durai, M., Linford, T. (2017). A state-of-the-art review: personalization of tinnitus sound therapy. Front. Psychol. 8, 1599. doi: 10.3389/fpsyg.2017.01599
Searchfield, G. D., Morrison-Low, J., Wise, K. (2007). Object identification and attention training for treating tinnitus. Progress Brain Res. 166, 441–460. doi: 10.1016/S0079-6123(07)66043-9
Searchfield, G. D., Sanders, P. J. (2022). A randomized single-blind controlled trial of a prototype digital polytherapeutic for tinnitus. Front. Neurol. 13, 958730. doi: 10.3389/fneur.2022.958730
Searchfield, G. D., Sanders, P. J., Doborjeh, Z., Doborjeh, M., Boldu, R., Sun, K., et al. (2021a). A state-of-art review of digital technologies for the next generation of tinnitus therapeutics. Front. Digit. Health 3, 724370. doi: 10.3389/fdgth.2021.724370
Searchfield, G. D., Zhang, J., Biswas, R., De Ridder, D., Deutsch, B., Hall, D., et al. (2021b). Emerging topics in the behavioral neuroscience of tinnitus. Cur. Top. Behav. Neurosci. 51, 461–48. doi: 10.1007/7854_2020_217
Shekhawat, G. S., Searchfield, G. D., Stinear, C. M. (2013). Role of hearing AIDS in tinnitus intervention: a scoping review. J. Am. Acad. Audiol. 24, 747–762. doi: 10.3766/jaaa.24.8.11
Shi, Y., Robb, M. J., Michaelides, E. M. (2014). Medical management of tinnitus: role of the physician. J. Am. Acad. Audiol. 25, 23–28. doi: 10.3766/jaaa.25.1.3
Simoes, J. P., Neff, P., Schoisswohl, S., Bulla, J., Schecklmann, M., Harrison, S. S., et al. (2019). Towards personalized tinnitus treatment: an exploratory study based on internet crowdsensing. Front. Public Health 7, 157. doi: 10.3389/fpubh.2019.00157
Simoes, J. P., Neff, P. K. A., Langguth, B., Schlee, W., Schecklmann, M. (2021). The progression of chronic tinnitus over the years. Sci. Rep 11, 4162. doi: 10.1038/s41598-021-83068-5
Simonetti, P., Oiticica, J. (2015). Tinnitus neural mechanisms and structural changes in the brain: the contribution of neuroimaging research. Int. Arch. Otorhinolaryngol. 19, 259–265. doi: 10.1055/s-0035-1548671
Smith, S. S., Kitterick, P. T., Scutt, P., Baguley, D. M., Pierzycki, R. H. (2021). An exploration of psychological symptom-based phenotyping of adult cochlear implant users with and without tinnitus using a machine learning approach. Prog. Brain Res. 260, 283–300. doi: 10.1016/bs.pbr.2020.10.002
Song, J., Lee, D., Choi, I., Lee, K. (2017). Objective diagnosis of tinnitus using resting-state EEC big data-based support vector machine learning. J. Hear. Sci. 1333.
Szczepek, A. J., Haupt, H., Klapp, B. F., Olze, H., Mazurek, B. (2014). Biological correlates of tinnitus-related distress: an exploratory study. Hear Res. 318, 23–30. doi: 10.1016/j.heares.2014.10.007
Taylor, J. A., Thompson, D. M., Hall, D. A., Walker, D.-M., McMurran, M., Casey, A., et al. (2020). The TinMan study: feasibility trial of a psychologically informed, audiologist-delivered, manualised intervention for tinnitus. Int. J. Audiol. 59, 905–914. doi: 10.1080/14992027.2020.1788730
Theodoroff, S. M., Schuette, A., Griest, S., Henry, J. A. (2014). Individual patient factors associated with effective tinnitus treatment. J. Am. Acad. Audiol. 25, 631–643. doi: 10.3766/jaaa.25.7.2
Traeger, A. C., Henschke, N., Hubscher, M., Williams, C. M., Kamper, S. J., Maher, C. G., et al. (2016). Estimating the risk of chronic pain: development and validation of a prognostic model (PICKUP) for patients with acute low back pain. PLoS Med. 13, e1002019. doi: 10.1371/journal.pmed.1002019
Tunkel, D. E., Bauer, C. A., Sun, G. H., Rosenfeld, R. M., Chandrasekhar, S. S., Cunningham, E. R., et al. (2014). Clinical practice guideline: tinnitus. Otolaryngol. Head Neck Surg. 151, S1–S40. doi: 10.1177/0194599814538403a56
Tyler, R., Ji, H., Perreau, A., Witt, S., Noble, W., Coelho, C. (2014). Development and validation of the tinnitus primary function questionnaire. Am. J. Audiol. 23, 260–272. doi: 10.1044/2014_AJA-13-0014
Tzounopoulos, T., Balaban, C., Zitelli, L., Palmer, C. (2019). Towards a mechanistic-driven precision medicine approach for tinnitus. J. Assoc. Res. Otolaryngol. 20, 115–131. doi: 10.1007/s10162-018-00709-9
Van de Heyning, P., Gilles, A., Rabau, S., Van Rompaey, V. (2015). Subjective tinnitus assessment and treatment in clinical practice: the necessity of personalized medicine. Curr. Opin. Otolaryngol. Head Neck Surg. 23, 369–375. doi: 10.1097/MOO.0000000000000183
Van der Wal, A., Luyten, T., Cardon, E., Jacquemin, L., Vanderveken, O. M., Topsakal, V., et al. (2020). Sex differences in the response to different tinnitus treatment. Front. Neurosci. 14, 422. doi: 10.3389/fnins.2020.00422
Vanneste, S., Joos, K., De Ridder, D. (2012). Prefrontal cortex based sex differences in tinnitus perception: same tinnitus intensity, same tinnitus distress, different mood. PLoS ONE 7, e31182. doi: 10.1371/journal.pone.0031182
Vanneste, S., Song, J. J., De Ridder, D. (2018). Thalamocortical dysrhythmia detected by machine learning. Nat. Commun. 9, 1103. doi: 10.1038/s41467-018-02820-0
Veile, A., Zimmermann, H., Lorenz, E., Becher, H. (2018). Is smoking a risk factor for tinnitus? a systematic review, meta-analysis and estimation of the population attributable risk in Germany. BMJ open. doi: 10.1136/bmjopen-2017-016589
Vielsmeier, V., Santiago Stiel, R., Kwok, P., Langguth, B., Schecklmann, M. (2020). From acute to chronic tinnitus: pilot data on predictors and progression. Front. Neurol. 11, 997. doi: 10.3389/fneur.2020.00997
Watts, E. J., Fackrell, K., Smith, S., Sheldrake, J., Haider, H., Hoare, D. J. (2018). Why is tinnitus a problem? a qualitative analysis of problems reported by tinnitus patients. Trends Hear. 22. doi: 10.1177/2331216518812250
Wise, K., Kobayashi, K., Magnusson, J., Welch, D., Searchfield, G. D. (2016). Randomized controlled trial of a perceptual training game for tinnitus therapy. Games Health J. 5, 141–149. doi: 10.1089/g4h.2015.0068
Keywords: tinnitus, smarter solutions, innovative research, Audiology, Otology, state of the art
Citation: Searchfield GD (2023) Specialty grand challenge: Smarter solutions for tinnitus. Front. Audiol. Otol. 1:1101233. doi: 10.3389/fauot.2023.1101233
Received: 17 November 2022; Accepted: 10 January 2023;
Published: 02 February 2023.
Edited and reviewed by: Anil K. Lalwani, Columbia University, Vagelos College of Physicians and Surgeons, United States
Copyright © 2023 Searchfield. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grant D. Searchfield,  g.searchfield@auckland.ac.nz
g.searchfield@auckland.ac.nz
 Grant D. Searchfield
Grant D. Searchfield