Non-thermal plasma discharge inside a sealed bag to sterilize and to preserve the sterile state of a medical device
-
1
University of Reims-Champagne-Ardenne, EA 4691 "Biomatériaux et Inflammation en Site Osseux", France
-
2
University of Orleans, UMR 7344 Groupe de Recherches sur l’Energétique desMilieux Ionisés, France
-
3
CRITT-MDTS, France
-
4
SOMINEX, France
Introduction: Nowadays sterilization of medical devices is most commonly achieved by high pressure saturated steam, ethylene oxide treatment, or by ionizing radiation. However, these techniques can severely damage thermosensitive medical devices or need a long ventilation periods before the use of the sterilized items[1],[2].
New techniques using non-thermal plasma have demonstrated the effectiveness of plasma ionization on the inactivation of various bacterial strains such as Pseudomonas aeruginosa…[3]-[5]Plasma is an ionized gas consisting of UV photons and reactive species with sufficient energy to alter bacteria[6],[7]. Nowadays if these techniques can sterilize thermosensitive items, it is not possible to maintain their sterile state after the end of the sterilization process[7].
The aim of this study was to demonstrate the feasibility of performing a non-thermal sterilization plasma discharge inside a sealed bag to preserve the sterile state of the items.
Materials and Methods: A prototype performing a plasma discharge inside a sealed bag has been developed (Patent: PTC/FR2011/05219)
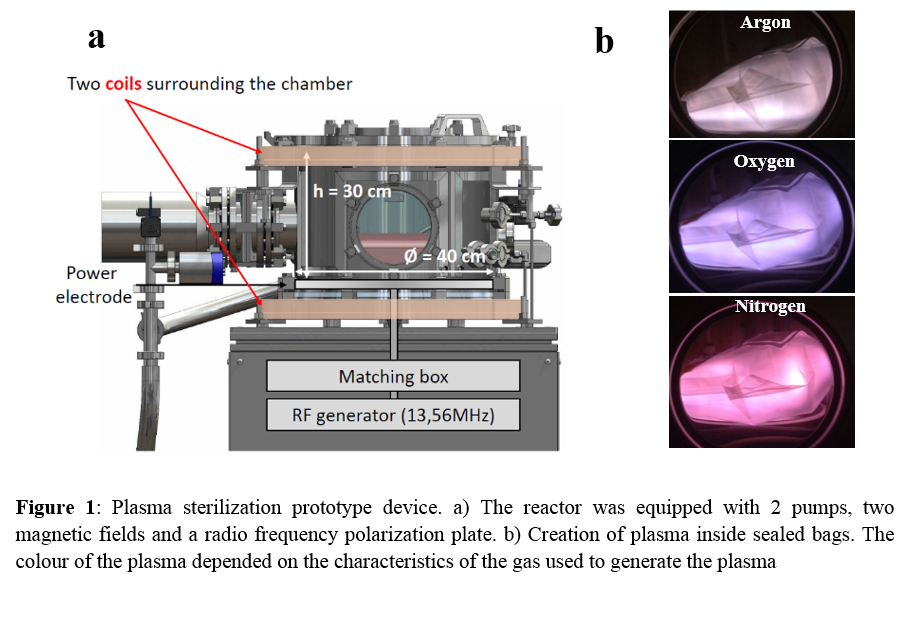
. The efficiency of this plasma sterilization process was evaluated on Pseudomonas aeruginosa and Staphylococcus aureus. Inoculated glass slides were placed in a sterilization bag which was sealed. The bag was exposed either to O2, N2 or Ar plasma treatments for varying exposure times from 5 to 120 min. Bacteria were counted to evaluate the bactericidal effect according to EN 1040 European Norm.
SEM observations of the bacteria morphology were observed before and after plasma treatments.To understand the process of ionized gases on bacteria, a characterization of O2, N2 or Ar plasmas was done by Optical Emission Spectroscopy from VUV to IR wavelengths (110-1100 nm). Plasma density was estimated by Microwave Interferometry from 2.108 cm-3 to 3.109 cm-3 depending on the gas and the RF power.
Results: Bacterial results clearly showed the potential of the prototype to sterilize pre-conditioned items inside a sealed bag. Regardless of the gas nature, inactivation of P. aeruginosa was obtained within a 45 min-plasma treatment. S. aureus appeared more resistant than P. aeruginosa and needed twice as much time to reach a bactericidal effect according to EN 1040 European Norm.
The morphology of the bacteria before and after plasma treatments was visualized by SEM. After a 45 min-plasma treatment (N2, Ar, O2), only fragments or damaged P. aeruginosa were visualized while a 120 min-plasma treatment was needed to alter S.aureus.
Plasma characterization by Optical Emission Spectroscopy has shown the production of intense UV radiations (210-280 nm), reactive species (OH, NO, O, N, H) and ions (N2+) in the three gases. VUV/UV photons are well-known to alter DNA whereas reactive species could alter bacteria membranes.
Conclusion: This plasma process covered by the patent PTC/FR2011/052199 could generate plasma directly in a sealed bag, leading to a sterilization process ensuring the medical industry sterility assurance level. Generation of UV photons and reactive species play a crucial part of the bacteria inactivation. Moreover this technique offers different advantages such as a process always above 40°C and the absence of toxic gases generation.
References:
[1] Holyoak GR, Wang S, Liu Y, Bunch TD. 1996. Toxic effects of ethylene oxide residues on bovine embryos in vitro. Toxicology, 108(1-2):33-8.
[2] Zhang YZ, Bjursten LM, Freij-Larsson C, Kober M, Wesslén B. 1996. Tissue response to commercial silicone and polyurethane elastomers after different sterilization procedures. Biomaterials, 17(23):2265-72.
[3] Alkawareek MY, Gorman SP, Graham WG, Gilmore BF. 2013. Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. Int J Antimicrob Agents, 43(2):154-60
[4] Kawamura K, Sakuma A, Nakamura Y, Oguri T, Sato N, Kido N. 2012. Evaluation of bactericidal effects of low-temperature nitrogen gas plasma towards application to short-time sterilization. Microbiol Immunol, 56(7):431-40.
[5] Lerouge S, Wertheimer MR, Marchand R, Tabrizian M, Yahia L. 2000. Effect of gas composition on spore mortality and etching during low-pressure plasma sterilization. J Biomed Mater Res, 51(1):128-35.
[6] Moisan M, Boudam K, Carignan D, Kéroack D, Levif P, Barbeau J, Séguin Y, Kutasi K, Elmoualij B, Thellin O, Zorzi W. 2013. Sterilization/disinfection of medical devices using plasma: the flowing afterglow of the reduced-pressure N2-O2 discharge as the inactivating medium. Eur Phys J Appl Phys, 63: 10001.
[7] Moisan M, Barbeau J, Crevier MC, Pelletier J, Philip N, Saoudi B. 2002. Plasma sterilization. Methods and mechanisms. Pure Appl Chem, 74(3): 349–358.
Keywords:
Bacteria,
Infection,
biomaterial,
device
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
General Session Oral
Topic:
Infection and its control in clinical biomaterials and devices
Citation:
Gelle
M,
Ben Belgacem
Z,
Charpentier
E,
Maho
T,
Robert
E,
Boudifa
M,
Pouvesle
J,
Polidor
F,
Laurent-Maquin
D and
Gangloff
S
(2016). Non-thermal plasma discharge inside a sealed bag to sterilize and to preserve the sterile state of a medical device.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.00151
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.