Introduction: Biomaterials with an organic and inorganic phase have been produced associated with carriers that can interact with human cells. The bacterial cellulose (BC) has been used for medical applications, since is an inert material, biodegradable, has elevated swelling degree and controlled porosity, which can act as an excellent carrier. Hydroxyapatite (HAp) is an inorganic material present on the bones, with high biocompatibility and it has been explored for metal adsorption. Strontium is a metal with similar properties to calcium, having similar action in the bone formation process on the human body. The present study aims to produce a hybrid material (HM) composed by BC and HAp and functionalize it with strontium, evaluating its adsorption and desorption properties.
Materials and Methods: The HM was obtained from bacterial cellulose associated to HAp, which was obtained by cyclic immersion in NaHPO4 and CaCl2 solutions. The hybrid adsorbent was characterized by SEM, FTIR, DSC and its swelling degree using water or phosphate buffer solution. Batch adsorption experiments were carried out for strontium impregnation onto material structure, under stirring and quantified by Atomic Absorption Spectrometry (AAS). The amount of Sr adsorbed on HM was quantified and the adsorption isotherm was modeled by Langmuir equation. Parameters as adsorbent amount, pH and temperature were investigated on Sr adsorption. The desorption process was evaluated using sodium phosphate buffer as elutant at pH 7.4, under stirring.
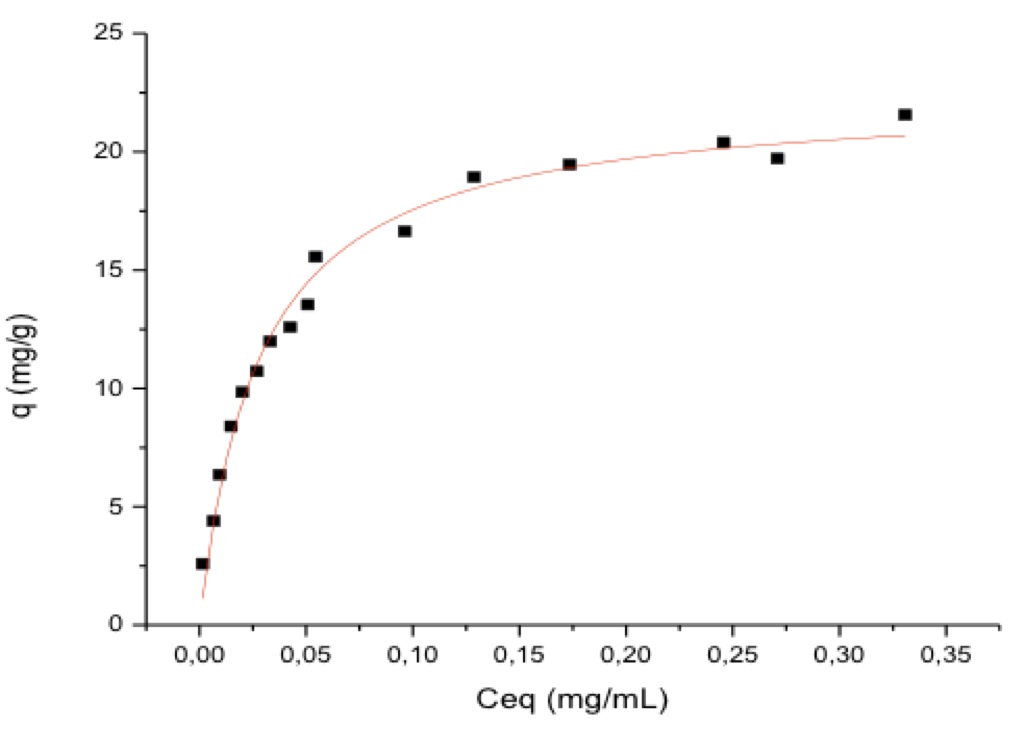
Results and Discussion: Figure 1 depicts the SEM images, showing the uniformity of cellulose fibers and HAp deposition on CB matrix. FTIR results confirmed that HAp interactes with the main functional groups of cellulose, mainly the hydroxyl, and there is the appearance of phosphate groups. DSC results showed exothermic character performing thermal degradability around 150 ° C. The material showed a high swelling degree either in water and in buffer solution. The equilibrium adsorption was reached in two hours, at pH 6.0.The equilibrium isotherm adsorption, modeled by Langmuir equation, showed that the maximum adsorption capacity was 22.42 ± 0.49 mg/g (Figure 2). Adsorbent amount, pH and temperature, showed that these parameters has influence on Sr adsorption and they are directly proportional The desorption results showed that in three hours about 60% of strontium adsorbed was eluted by PBS.
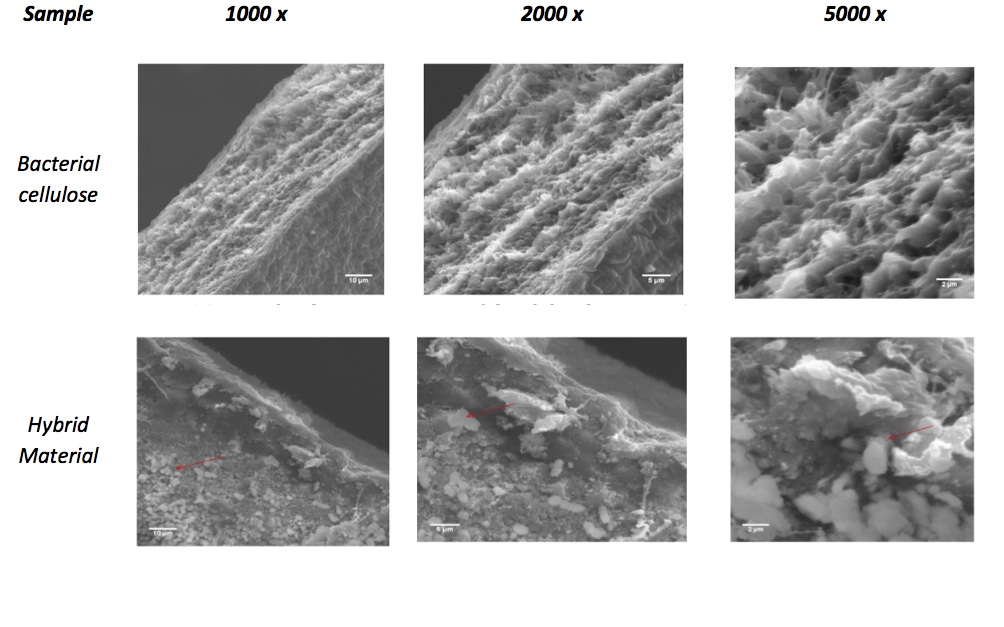
Conclusion: The HM presented an homogeneous surface, with HAp deposits on CB surface, with low porosity than raw CB. This biomaterial produced could remove soluble strontium from aqueous solution, probably by interaction (chemical or physical) between main functional groups from CB and HAp. The desorption results showed about 60% of strontium was completely released within 30 minutes, using phosphate buffer as eluent. This phenomenon could be attributed to the physical adsorption, which is more weakly, demonstrating that some Sr species are chemically adsorbed.
CNPq; CAPES; Central Analítica UFC