Summary: Collagen, the most abundant protein in mammals, plays a crucial role in tissue development and regeneration. Since elevated activity of collagen remodeling is associated with numerous pathologic conditions (e.g. tumor, fibrosis, arthritis), ability to target unstructured collagens in diseased tissue could lead to new diagnostics and therapeutics, as well as applications in regenerative medicine. Previously, we reported the new strategy for targeting denatured collagens that is based on triple helical hybridization between collagen strands (of diseased tissues) and synthetic collagen mimetic peptide (CMP) also referred to as collagen hybridizing peptide (CHP). This hybridization results in robust collagen specific binding in vivo which allows detection of degraded collagens in diseased tissues with persistent wound healing activity (e.g. tumor, arthritis)[1],[2].
In this presentation we will describe our on going investigations into elucidating the mechanism of the hybridization as well as experiments verifying the CHP’s collagen binding capacity both in vitro and in vivo (Fig. 1).
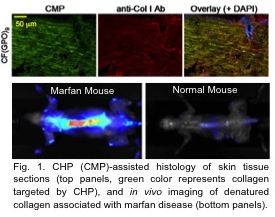
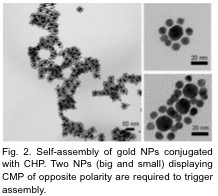
We will also discuss two new CHP-mediated self-assembling systems which are based on nanoparticles and beta-sheet forming peptides (B-CMPs). CHP conjugated nanoparticles (Fig. 2) allow controlled NP assembly as well as specific detection and removal of denatured collagens from protein mixtures.
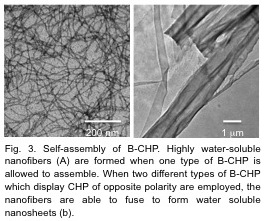
B-CHPs based on FKFE and GPO repeats assemble into highly water-soluble nanofibers and nanosheets (Fig. 3) which exhibit improved affinity to denatured collagen because of multi-ligand effects.
Due to its neutral and hydrophilic nature, CHPs are inert peptides exhibiting high serum stability. It is also a structurally simple peptide that can be readily conjugated to various imaging and therapeutic modalities. The CHP offers an entirely new way to target the microenvironment of diseased tissues which may lead to new opportunity for management of pathologic conditions associated with high level of collagen degradation and remodeling.
This work was supported by grants from NIAMS/NIH (R01-AR060484 and R21-AR065124) and DOD (W81XWH-12-1-0555).
References:
[1] Y. Li, C. A. Foss, D. D. Summerfield, Jefferson J. Doyle, Collin M. Torok, Harry C. Dietz, M. G. Pomper and S. M. Yu (2012) Targeting Collagen Strands by Photo-Triggered Triple Helix Hybridization. Proc. Nat. Acad. Sci. USA, 109, 14767.
[2] Y. Li, and S. M. Yu (2013) Targeting and mimicking collagens via triple helical peptide assembly. Curr. Opin. Chem. Biol. 17, 968.