Introduction: Success of personalized medicine in cancer therapy depends on the ability to molecularly phenotype tumors. Current clinical imaging techniques cannot be integrated with precision molecular medicine at the level of single cells or microlesions due to limited resolution. Our innovative optical imaging approach utilizes rare earth (RE) nanoparticles that absorb near infrared (NIR) radiation and emit in the shortwave infrared (SWIR) spectrum (1000-3000 nm), allowing for greater depth of detection than comparable contrast agents, such as quantum dots and carbon nanotubes. RE probes can be encapsulated in human serum albumin to generate biocompatible nanocomposites (REANCs) that can be decorated with a variety of ligands and therapeutic payloads for targeted imaging[1],[2]. In this study, we show CXCR4-targeting REANCs (fReANCs) to preferentially accumulate in tumor lesions and detect deep (~ 1 cm) tissue lesion dynamics with more sensitivity and specificity than existing preclinical imaging modalities such as whole body bioluminescence imaging (BLI).
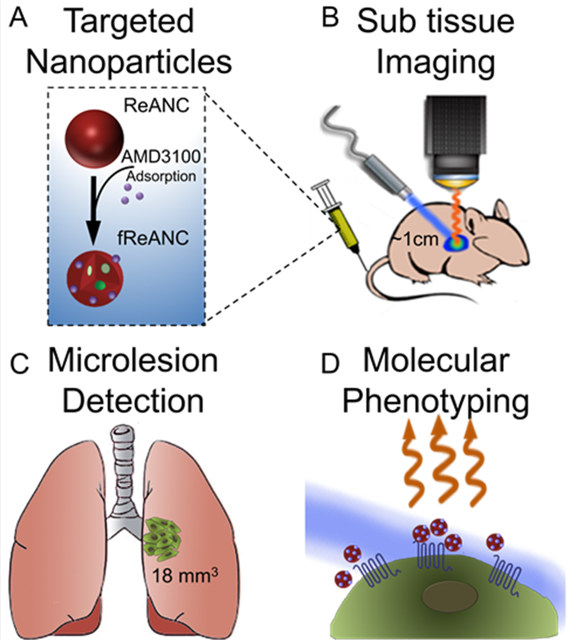
Longitudinal detection and molecular mapping of tumors is particularly promising in metastatic cancer biology where adequate imaging tools to map evolving heterogeneity leading to informed therapy decisions is an unmet need.
Materials and Methods: REANCs were synthesized and functionalized as published[3]. Their ability to detect micro-lesions in vitro was evaluated using cellular microclusters generated by incubating MDA-MB-231 metastatic breast cancer cells with ReANCs. The minimum threshold for detection of micro-clusters was compared between REANCs, luciferase, QDs, and carbon nanotubes and quantified. To validate the constructs’ ability to detect micro-metastatic lesions in internal organs such as lungs, female athymic nude mice were inoculated with luciferase labeled MDA-MB-231 derived cell line via their tail vein. ReANCs were administered i.v. once a week and SWIR imaging performed up to 24 hours post injection and compared to detection by BLI.
Results and Discussion: SWIR imaging was able to resolve cell clusters of fewer than 100,000 cells, in vitro. The sensitivity of detection of micro-clusters was compared to standard contrast agents and found to be higher. The minimum threshold for detection for all systems were compared and quantified. In vivo, SWIR imaging was able to resolve micro-metastatic subcutaneous lesions that were only millimeters in length and width. Longitudinal in vivo imaging confirmed preferential accumulation of fREANCs in tumor lesions compared to control ReANCs in our lung metastases models. Additionally, we were able to detect lesions up to 1cm into the animal’s tissue and as small as 18.9mm^3 in volume as validated by MRI. Specifically, we were able to demonstrate a superior preclinical imaging modality with greater depth of penetration and better resolution capable of detecting early micro-metastatic lesions.
Conclusion: Findings from this study support the promise of the “new window” imaging platform and suggest future clinical translatability for nanomedicine. Our novel nanoprobes with multi-spectral and multi-functional potential will pave the way for longitudinal imaging of lesion mapping and detecting evolving molecular changes. This novel imaging paradigm will not only play a role in precision medicine but also in surgical areas where image guidance will ensure clean margins for better prognosis.
NIH R01 EB018378 (Moghe, Roth, Riman)
References:
[1] Ming, X.; Carver, K.; Wu, L., Biomaterials 2013, 34 (32), 7939-49.
[2] Naczynski, D.; Andelman, T.; Riman, R..; Roth, C..; Moghe, P. Small 2010, 6, 1631-40.
[3] Naczynski, D. J.; Tan, M. C.; Zevon, M.; Kulesa, A.; Chen, S.; Roth, C. M.; Riman, R. E.; Moghe, P. V., Nature communications 2013, 4, 2199. DOI 10.1038/ncomms3199.