Introduction: Sustained delivery of insulin is crucial for many patients with diabetes. Maintaining a basal level of insulin is currently achieved by daily injections of long-acting insulin[1]. However, frequent injections pose a problem in patient compliance, so there has been interest in developing an injectable subcutaneous depot that slowly releases insulin over time[2]. The goal is to have a highly biocompatible and biodegradable system suitable for regular injections, and the naturally occurring hyaluronic acid is an ideal platform to work on[3]. Here, we report the formation of hyaluronic acid hydrogels via cyclodextrin host-guest interactions, and investigate this delivery system for the sustained release of insulin in vitro.
Materials and Methods: Hyaluronic acid modified with adamantane (Ad-HA) and poly-β-cyclodextrin were loaded with insulin and mixed at stoichiometrically equivalent ratios to form a hydrogel. In vitro release assays were performed in PBS buffer and at 37 degrees Celsius.
Results: When Ad-HA is mixed with β-cyclodextrin polymer, a relatively soft and sticky hydrogel forms rapidly. Insulin was loaded in the hydrogel, and studied for its in vitro release properties. Loading insulin in the hydrogel prolongs insulin release significantly compared to insulin solution, and hydrogel-loaded insulin can be released almost linearly for about 2 days. When a single release curve is log transformed, we can obtain the gradient and y-intercept which give a summary description of the release profile.
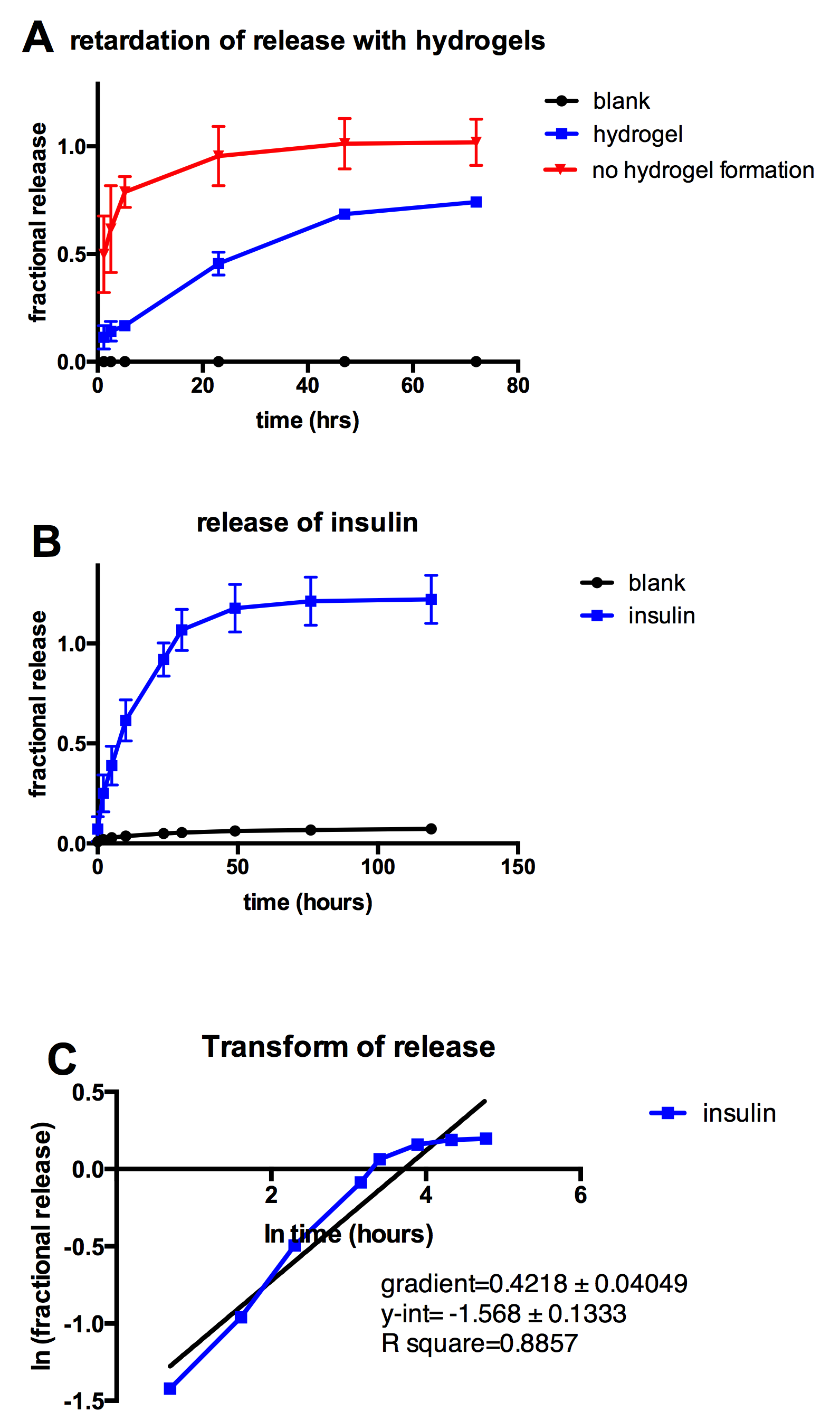
Fig. Release of insulin from the host-guest hydrogel. A. Retardation of insulin release with the presence of the hydrogel. B. Release of insulin over time. C. Log transformation of B to get numerical values for the gradient, which can be used to summarise release experiments.
Discussion: Hyaluronic acid hydrogels have been synthesized and developed for delivery of the small peptide insulin. These hyaluronic acid hydrogels can release the small peptide insulin for 2 days with an almost linear release. Even though the release seems fast, the hydrogel is at a much lower weight percent than regular PEG hydrogels or block copolymer hydrogels, and the hyaluronic acid base material is more biodegradable. The results show sustained release of insulin, and we believe that this is a firm foundation for further work on modifying hydrogel parameters and excipients to achieve a week-long sustained release system. The release profile of insulin can be described by the gradient and y-intercept after log transformation, and this provides a simple release descriptor for screening of many different formulation conditions.
Conclusion: Shear-thinning hyaluronic acid hydrogels have been developed as an injectable depot for the sustained delivery of the small peptide insulin. These results are an important step towards a week-long sustained delivery of insulin or other labile drugs. The usage of hyaluronic acid as a base material offers the possibility of a fast degrading delivery depot that can degrade and be reabsorbed shortly after the drug has all been released.
References:
[1] Rosenstock J, et al. (2001) Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 24(4):631–636
[2] Berenson, D.F., Weiss, A.R., Wan, Z.L., Weiss, M.A. (2012) Insulin analogs for the treatment of diabetes mellitus: therapeutic applications of protein engineering. Annals of New York Academy of Sciences, 1243: E40–E54
[3] Burdick, J.A., Prestwich, G.D. (2011) Hyaluronic Acid Hydrogels for Biomedical Applications. Advanced Materials, 23:H41-H56