Introduction: Recent progress in 2D nanomaterials technologies has paved the way towards the development of advanced biomaterials[1]-[3]. In the large family of 2D nanomaterials, exfoliated synthetic clays have been used in many advanced technological applications such as hydrogel nanocomposites with self-healing properties[4], injectable hemostatic biomaterials[5], and as bioactive material for bone tissue engineering[6]. Engineered bioactive hydrogels can mimic the properties of the extracellular matrix due to their structural similarity to the native extracellular matrix[6],[7]. However, these materials present insufficient in-vivo degradation which results in slow resorption. They also have limited injectability, and tissue regeneration capabilities, making necessary the development of a new biomaterial that is injectable and can facilitate bone formation.
Magnesium plays an important role in mineral metabolism promoting calcification, hydroxyapatite crystal formation, bone cell adhesion, proliferation, and differentiation. Among phosphate-based materials, magnesium phosphates have demonstrated to be biocompatible and resorbable in-vivo making them interesting for biomedical applications[8],[9]. Thus, biomaterials made of magnesium phosphate can be used for bone tissue engineering. However, there is no biomaterials made of magnesium phosphate with osteoinductive, injectability, and hydrogel-like features such as these found in 2D-nanomaterials.
Here, we develop a magnesium phosphate material with 2D morphology that was synthesized by tuning the crystallization of the sodium-magnesium-phosphate system. This novel material is injectable and can enhance bone formation.
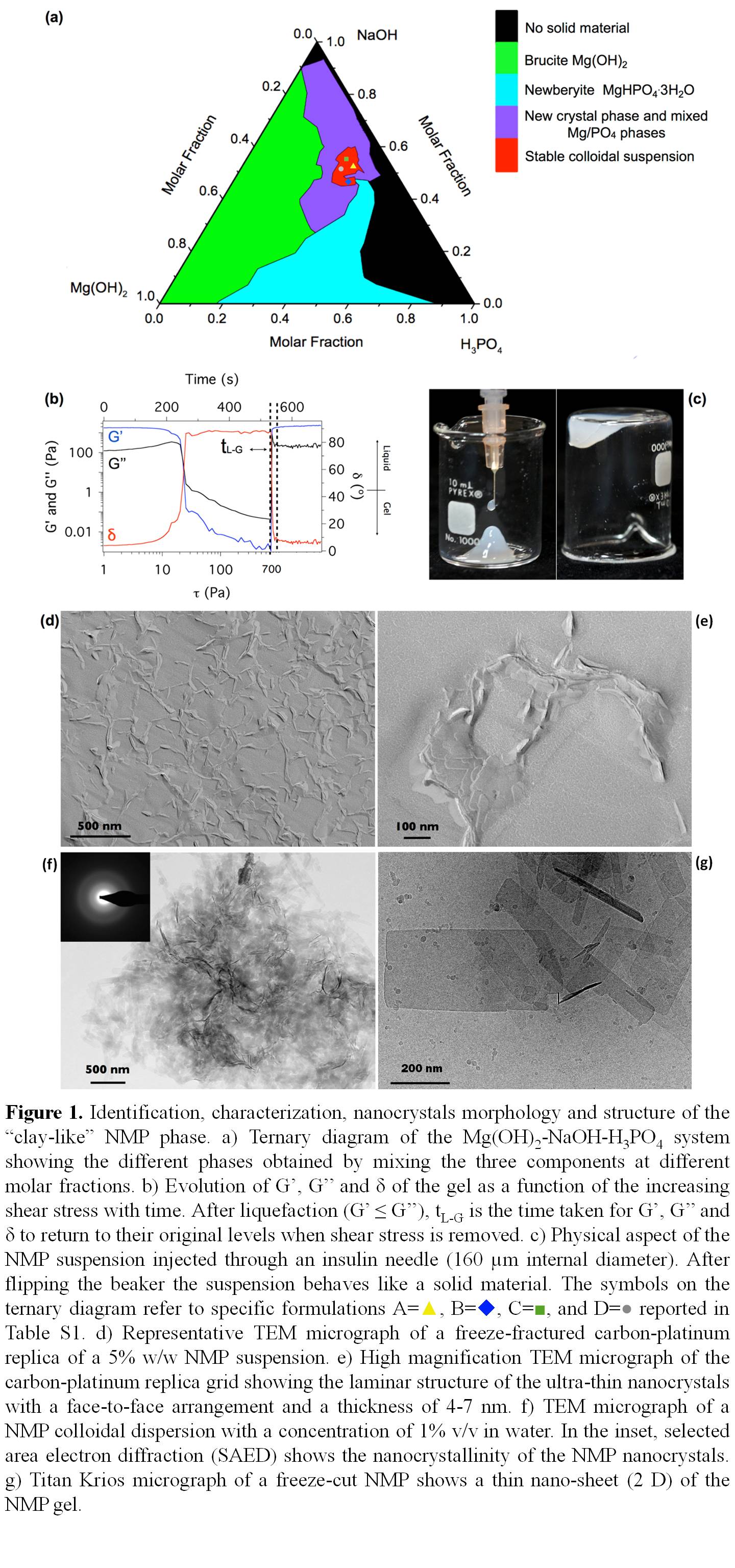
Methods: The 2D nanocrystalline magnesium phosphate (NMP) biomaterial was discovered by studying the ternary diagram of the system NaOH-Mg(OH)2-H3PO4, and it was obtained in a small region of the ternary diagram (Figure 1a, red area). NMP was characterized using transmission and focus ion beam scanning electron microscopy, Cryo-transmission electron microscopy (Titan-Krios), nuclear magnetic resonance (14 tesla), X-ray diffraction and rheology (figure 1).
Mouse-derived bone marrow cells (mBCMs) were used to assess the effects of NMP on in-vitro gene expression relevant to bone metabolisim; RunX2, ALP, COL1A1, OCN, and OPN.
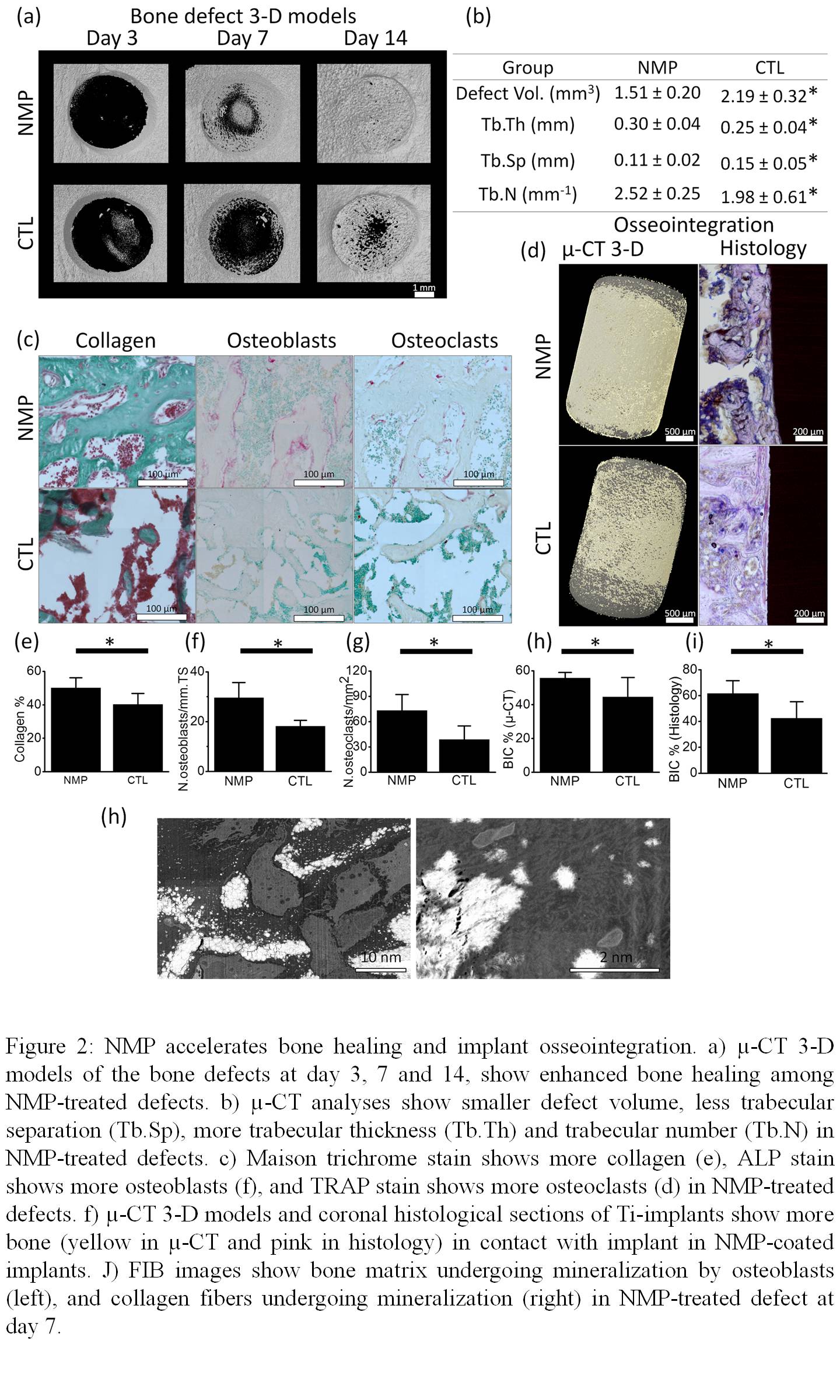
Bone healing and implant osseointegration were assessed in-vivo using a rat-tibiae-model (n=12). In each rat, a titanium implant was placed in the left tibia, and a bone defect was created in the right tibia. In the experimental animals (n=12) implants and bone defects were treated with the NMP, while the control animals (n=12), defects and implants were left untreated. Two weeks after the surgery, rats were euthanized and bone healing and osseointegration were assessed using µ-CT and histomorphometry. Osseointegration was assessed with µ-CT using a new method that we for this study.
Results and Discussion:
We discovered that the sodium ion can regulate the precipitation of magnesium phosphate by interacting with specific surfaces of the crystals causing a preferential crystal growth that results in 2D morphology. The 2D material gave rise to a physical hydrogel that presented long term stability, thixotropy, injectability, biocompatibility, and bioresorption. Furthermore, the material presented unique biological properties, it accelerates bone healing and osseointegration by enhancing osteoclasts proliferation and osteoblasts differentiation (figure 2) through up-regulation of COL1A1, RunX2, ALP, OCN and OPN.
Conclusions: The 2-D nano-material described here can be injected through high gauge needles into bone, to enhance bone formation, could bring a paradigm shift in the field of minimally invasive bone regeneration.
McGill University, Faculty of Dentistry; Saudi Arabian Cultural Bureau; University of Dammam, College of Dentistry; Canadian Institutes of Health Research; Network for Oral and Bone Health Research; Dr. Charles Doillon for providing the human fibroblast cells
References:
[1] Dawson, J. I. & Oreffo, R. O. Clay: new opportunities for tissue regeneration and biomaterial design. Advanced Materials 25, 4069-4086 (2013).
[2] Dawson, J. I., Kanczler, J. M., Yang, X. B., Attard, G. S. & Oreffo, R. O. Clay gels for the delivery of regenerative microenvironments. Advanced Materials 23, 3304-3308 (2011).
[3] Aguzzi, C., Cerezo, P., Viseras, C. & Caramella, C. Use of clays as drug delivery systems: possibilities and limitations. Applied Clay Science 36, 22-36 (2007).
[4] Wang, Q. et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 463, 339-343 (2010).
[5] Gaharwar, A. K. et al. Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS nano 8, 9833-9842 (2014).
[6] Xavier, J. R. et al. Bioactive Nanoengineered Hydrogels for Bone Tissue Engineering: A Growth-Factor-Free Approach. ACS nano 9, 3109-3118 (2015).
[7] Tibbitt, M. W. & Anseth, K. S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnology and bioengineering 103, 655-663 (2009).
[8] Tamimi, F. et al. Biocompatibility of magnesium phosphate minerals and their stability under physiological conditions. Acta biomaterialia 7, 2678-2685 (2011).
[9] Klammert, U., Ignatius, A., Wolfram, U., Reuther, T. & Gbureck, U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta biomaterialia 7, 3469-3475 (2011).