Introduction: The understanding of the pathomechanisms of age-related macular degeneration (AMD) remains a challenge. Typical features of the disease such as drusen and the role of growth factors have been extensively studied[1]-[3], but it remains unclear what causes drusen formation and a growth factor imbalance. Research is directed towards animal models[4] and more recently towards stem cell based therapies which are still in their infancy[5]. Monolayer culture is a widely used alternative to animal studies, but is limited by the relative lack of sophistication when compared to the human retina, since the pathogenesis of AMD involves complex interplay between the retinal pigment epithelium (RPE), Bruch’s membrane and the choroid. It is becoming apparent that 3D culture systems are better suited for complex disease models to allow the study of cellular crosstalk within the microenvironment with advanced co-culture system[6]. Therefore, this study aimed at developing a novel humanized trilayer culture model by culturing human fetal RPE and primate choroidal cells on electrospun medical-grade polycaprolactone (PCL)-gelatin meshes to study the initial changes in AMD.
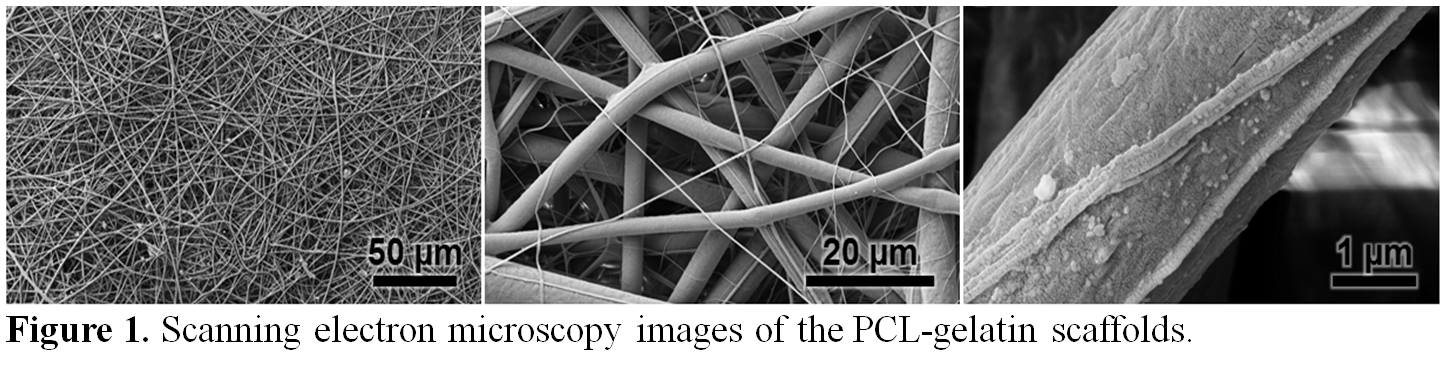
Materials and Methods: Electrospun PCL-gelatin meshes were made by a custom solution electrospinning apparatus as described previously[7] (Figure 1). The overall architecture of the scaffolds, including the porosity and the surface area-to-volume ratio, was analyzed using the nano-computed tomography (nano-CT) system using a resolution of 1 µm, a voltage of 45 kVp and a current of 177 mA (n=4). The porous nature of the scaffold was an important consideration in studying the effect of co-culture and the possible crosstalk between the RPEs and the choroidal cells. The trilayer model was prepared by seeding the primate choroid endothelial cell line (RF/6A) on the bottom surface of the PCL-gelatin scaffold coated with human recombinant laminin followed by seeding human RPE cells on the top surface of the scaffold[8] (ratio 1:1; 104 cells/cm2), mimicking the retina in vivo microenvironment.
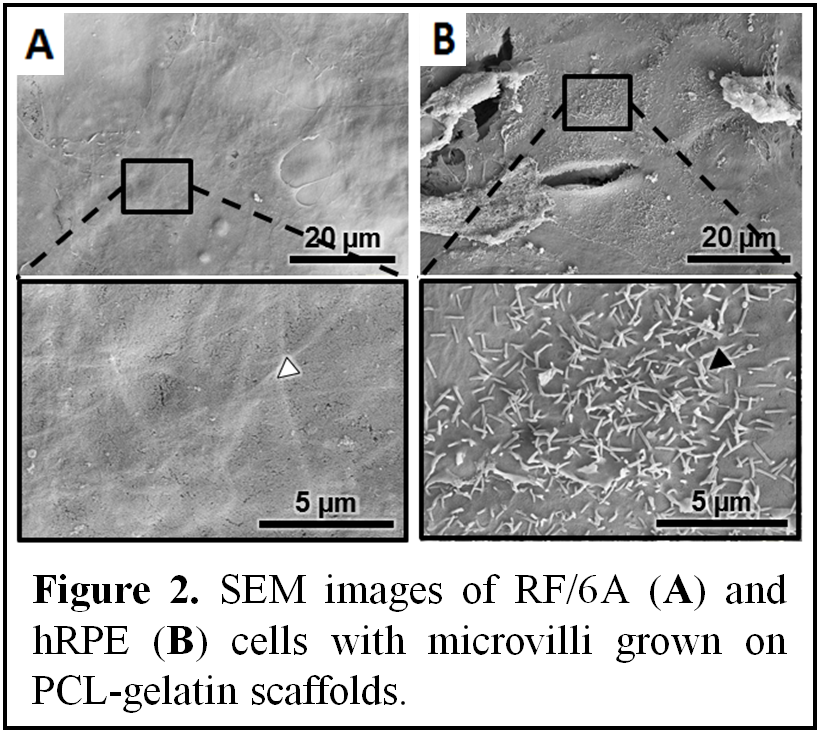
Results and Discussion: Scanning electron microscopy images confirmed that PCL-gelatin electrospun scaffolds allowed excellent cell attachment and infiltration (Figure 2). Functional and morphological analysis of the human RPE cells showed that they were able to phagocytose vitronectin-coated microbeads and that they formed microvilli (Figure 2B). The quantitative analysis of vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF), factors critical in pathophysiological condition of AMD, indicated significant increases in production of both VEGF and PEDF in RPE/choroidal co-cultures compared to mono-cultures. Our findings indicate the functionality of the current model and provide a strong argument into the advantages of using a 3D trilayer in vitro culture model to study early changes involving the shift in the PEDF/VEGF equilibrium that occurs in AMD.
Conclusion: In summary, we provide the initial evidence that a three dimensional in vitro model can be fabricated to exhibit functional characteristics typical for human RPE and choroidal cells. We believe that this novel bioengineered 3D construct can provide a platform for further studies in the pathomechanisms of AMD.
This study was funded by National Health and Medical Research Council (NHMRC).
References:
[1] Hageman, Gregory S., and Robert F. Mullins. "Molecular composition of drusen as related to substructural phenotype." Mol Vis 5.28 (1999): 28.
[2] Ohno‐Matsui, Kyoko, et al. "Novel mechanism for age‐related macular degeneration: An equilibrium shift between the angiogenesis factors VEGF and PEDF." Journal of cellular physiology 189.3 (2001): 323-333.
[3] Johnson, Lincoln V., et al. "Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration." Proceedings of the National Academy of Sciences 108.45 (2011): 18277-18282.
[4] Fletcher, Erica L., et al. "Age-Related Macular Degeneration: What’s New and on the Horizon." Optometry & Vision Science 91.8 (2014): 816-818.
[5] Nazari, Hossein, et al. "Stem cell based therapies for age-related macular degeneration: The promises and the challenges." Progress in retinal and eye research 48 (2015): 1-39.
[6] Feigl, Beatrix, and Dietmar Hutmacher. "Eyes on 3D‐Current 3D Biomimetic Disease Concept Models and Potential Applications in Age‐Related Macular Degeneration." Advanced healthcare materials 2.7 (2013): 1056-1062.
[7] Chong, E. J., et al. "Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution." Acta biomaterialia 3.3 (2007): 321-330.
[8] Hamilton, R. D., A. J. Foss, and L. Leach. "Establishment of a human in vitro model of the outer blood–retinal barrier." Journal of Anatomy 211.6 (2007): 707-716.