Introduction: Electrospinning is a process that uses the electrical to stretch a viscous solution or melt by the applied potential to form nanofibers. However, most current electrospinning process involves the usage of organic solvents and most of the solvents used are toxic and corrosive, which will bring serious environment problem. Therefore, “Green Electrospinning” was proposed as an approach of fabricating nanofibers by electrospinning from aqueous concentration[1]. The extracellular matrix (ECM) is a complex of structural and functional proteins, glycoproteins, and proteoglycans arranged in a unique, tissue specific three-dimensional ultrastructure[2],[3]. Gelatin is a protein biopolymer derived from partial hydrolysis of native collagens, which are the most abundant structural proteins found in the animal body of skin, tendon, cartilage and bone[4]. Pullulan is an extracellular linear polysaccharide produced by the fungus-like yeast, Aureobasidium pullulans[5]. In the present work, gelatin and pullulan were electrospun into nanofibers by electrospinning to mimic the extracellular matrix. The morphology of electrospun nanofibers was observed by SEM. The fiber diamter distribution was also discussed in this study.
Materials and Methods: Materials: Gelatin (Type A, 300 bloom) was purchased from Sigma-Aldrich Chemical Company (St. Louis, Missouri, USA). Pullulan at food grade was purchased from Hayashibara Biochemical Laboratories Inc. (Okayama, Japan). Doubly distilled water was used as a solvent to prepare all solutions. All products were received without further purification.
Electrospinning: Gelatin and pullulan were dissolved in doubly distilled water seperately at room temperature under magnetic stirring. The result solution was fed into plastic syringe. During electrospinning, a positive high voltage of 24 kV generated by a high voltage power supply (JDF-1, China) was applied at the tip of a syringe needle. The electrospun nanofibers were collected on a piece of aluminum foil covered on an electrically grounded metal plate, which was placed at a distance of 12 cm below the tip of the syringe needle. The mass flow rate was maintained at 0.6 ml/h by extremely accurate syringe pump (789100C, Cole-Parmer, USA). The electrospinning was conducted under the ambient conditions. The behavior of the pullulan solution during electrospinning was observed visually. The morphology of electrospun nanofibers were observed by SEM (JSM 6460, JEOL Ltd, Japan).
Results and Discussion: The gelatin and pullulan complex were successfully electrospun into nanofibers for aqueous solution, as shown in figure 1. The concentration or the corresponding viscosity was one of the most effective variables to control the fiber morphology. Meanwhile the weight ratio between gelatin and pullulan will also affect the size and morphology of diameter. As shown in figure 2, the average diameter was 152nm, and fiber diameter ranged from 100 to 200nm. Moreover, the interaction between gelatin and pullulan will further be investigated.
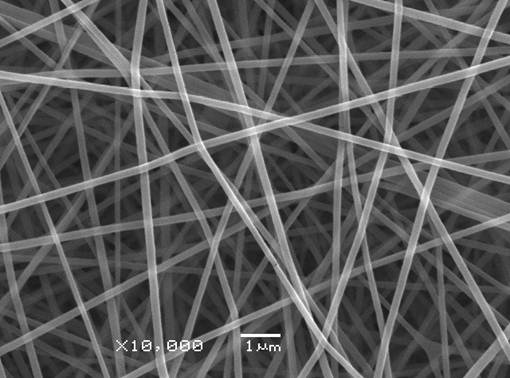
Figure1. SEM image of electrospun gelatin and pullulan nanofibers
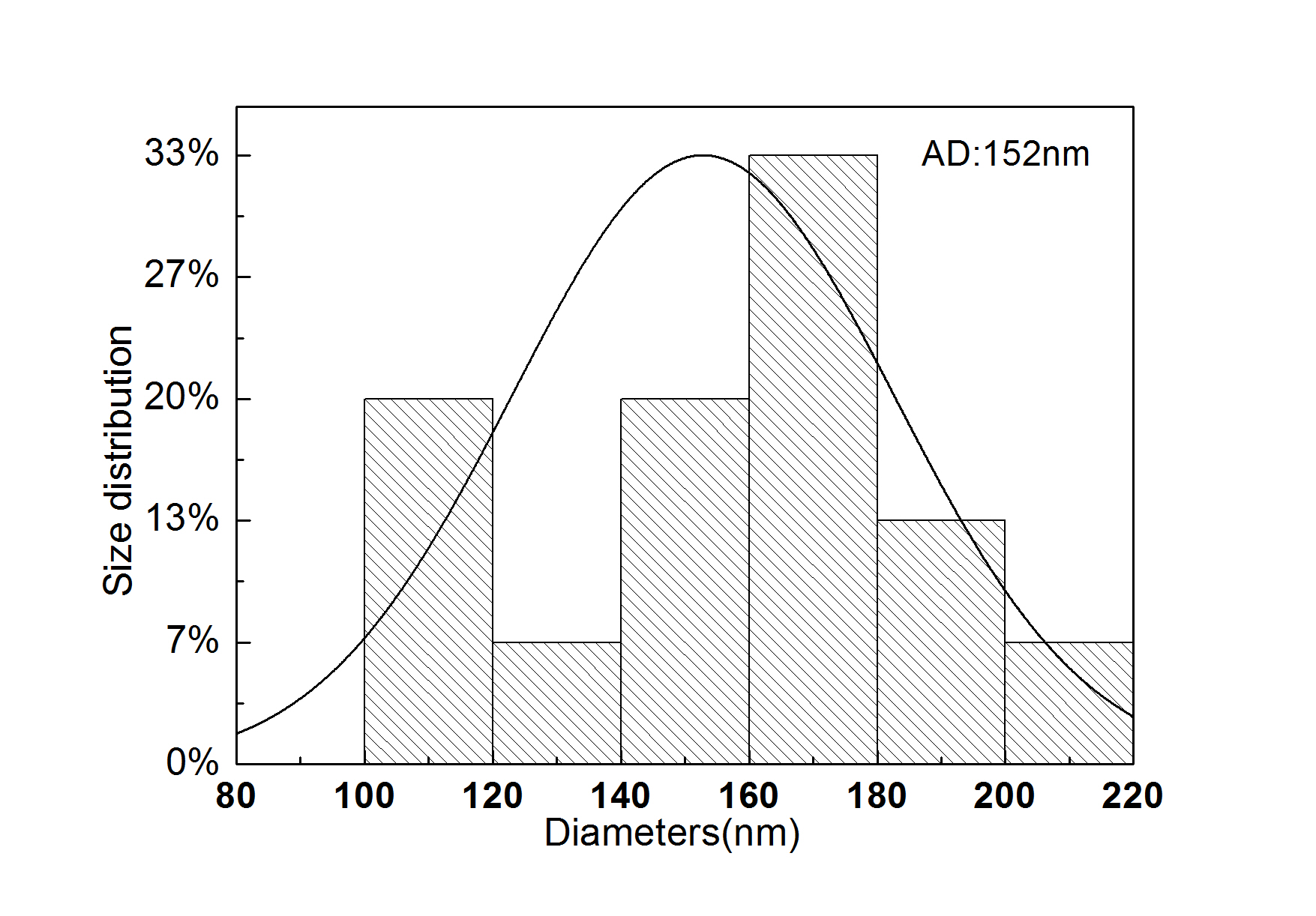
Figure2. Size distribution of electrospun gelatin and pullulan nanofibers
Conclusion: Gelatin and pullulan complex as a novel mimic system has been successfully electrospun into nanofibers from aqueous solution, which will avoid the harm of organic solvents. The new complex has potential application in tissue engineering scaffolds.
China Scholarship Concil
References:
[1] Jinyuan Sun, Kathrin Bubel, Fei Chen, et al, Nanofibers by Green Electrospinning of Aqueous Suspensions of Biodegradable Block Copolyesters for Applications in Medicine, Pharmacy and Agriculture. Macromolecular Radid Communication, 2010,31:2077-2083
[2] Bidylak SF, The extracellular matrix as a scaffold for tissue reconstruction. Seminars in Cell and Developmental Biology. 2002;13(5):377-383
[3] Huang GP, Shanmugasundaram S, Marih P. et al. An investigation of common crosslinking agents on the stability of electrospun collagen scaffolds. Journal of Biomedical Material Research, Part A, 2015,103A:762-771
[4] Ward AG, Courts A. The science and technology of gelatin. London: Academic Press, Inc., Ltd; 1977.
[5] Kachhawa D.K., Bhattacharjee P., Singhal R.S., Studies on downstream processing of pullulan. Carbohydrate Polymers, 2003,52:25-28