Introduction: Polymers based on methacrylic acid (MAA) were previously shown to have a therapeutic effect on wound closure through the promotion of angiogenesis of surrounding tissues[1]-[3]. However, it is unclear how this polymer exerts these beneficial effects.
The main goal of this current study is to understand and characterize the response of endothelial cells and macrophages to MAA-co-IDA copolymer film (IDA=isodecyl acrylate, in comparison to methyl methacrylate copolymer – MM-co-ID, used as a control) by identifying a critical signalling pathway, which is activated during cell-material interaction.
Materials and Methods: In the study, we have used the following cell types: 1) Phorbol myristate -acetate differentiated THP1 cells, and 2) mouse BMDM (bone marrow-derived macrophages), which were differentiated with MCSF and then IFNγ or IL-4 polarized to M(IFNγ) [M1] and M(IL-4) [M2] macrophages respectively, and 3) HUVEC (Human Umbilical Vein Endothelial Cells). dTHP1 were incubated with 40% MAA or 40% MM polymer films for 72h for gene expression analysis or for 24 hours to produce conditioned medium; HUVEC were incubated for 72h with the conditioned media. M0, M1 and M2 macrophages were incubated with 40% MAA or 40% MM polymer films for 72h.
Results and Discussion: MAA upregulated IGF-1 (Insulin like Growth Factor 1), but not IGF-2 (Insulin like Growth Factor 2) in dTHP1cells or mouse macrophages (Figure 1). It was hypothesized that IGF-1 in MAA-conditioned medium stimulates HUVEC.

Scratch wound assay in HUVEC indicated increased migration of the cells after their incubation with MAA-treated dTHP1 conditioned medium in comparison to MM-treated dTHP1 conditioned medium.
Gene expression analysis in dTHP1 and M0, M1 and M2 macrophages showed that MAA polymer increased the expression of IGFBP-3 (3.5-fold in dTHP1; 4.5-fold in M2 macrophages; 2.2-fold in M1 macrophages and 6.5-fold in M0 macrophages) relative to MM (Figure 2). IGFBP-3 is known to block IGF-1 access to the IGF-1 receptor and thereafter deliver more IGF1 into the blood stream[4],[5]. Macrophages also demonstrated decreased expression of IGF-R1 when treated with MAA polymer (Figure 2).
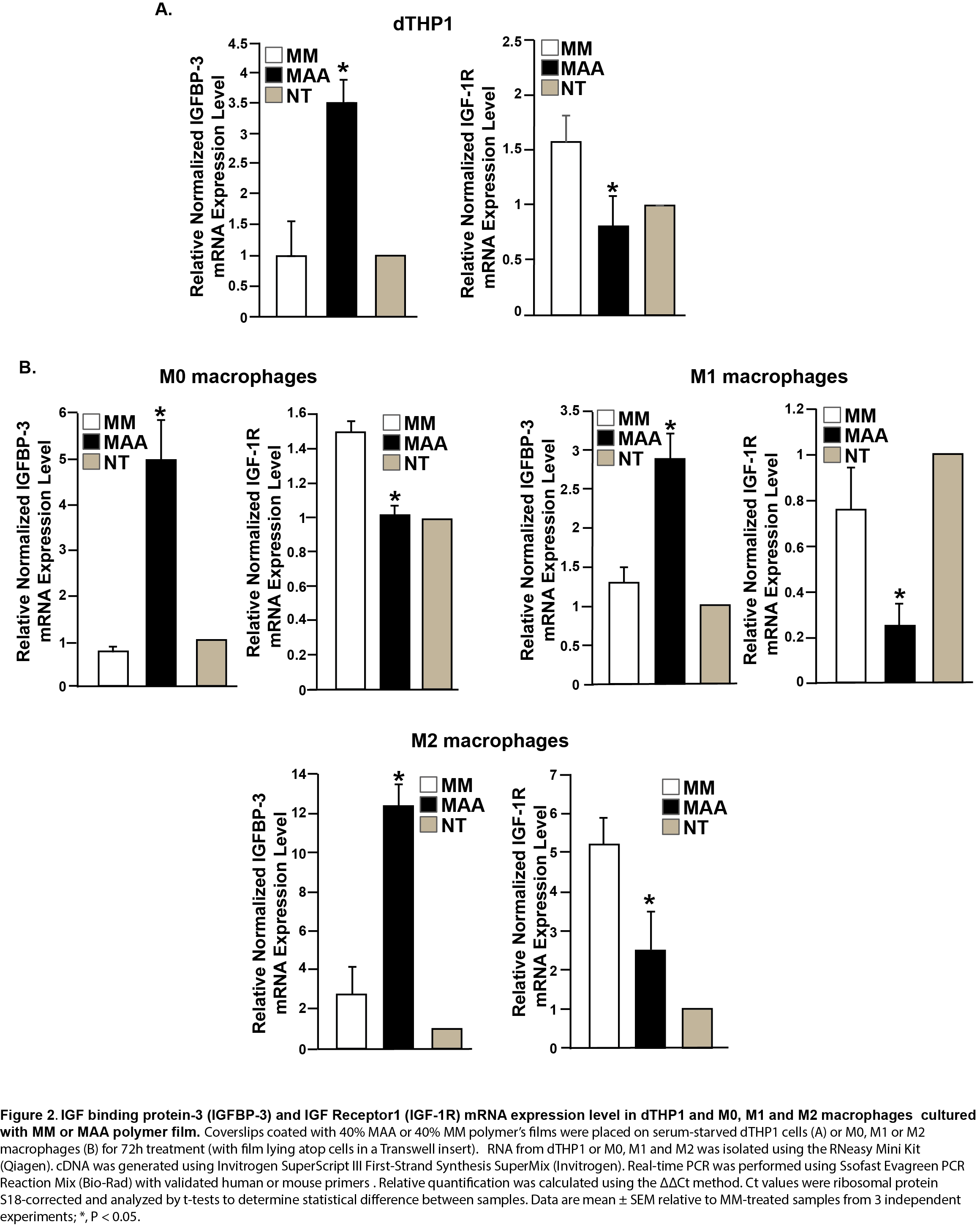
In contrast, HUVEC treated with MAA stimulated dTHP1 conditioned media showed increased expression of IGF-R1 (2-fold), but decreased expression of IGFBP-3, which is opposite to dTHP1 cells treated with MAA polymer film (Figure 3).
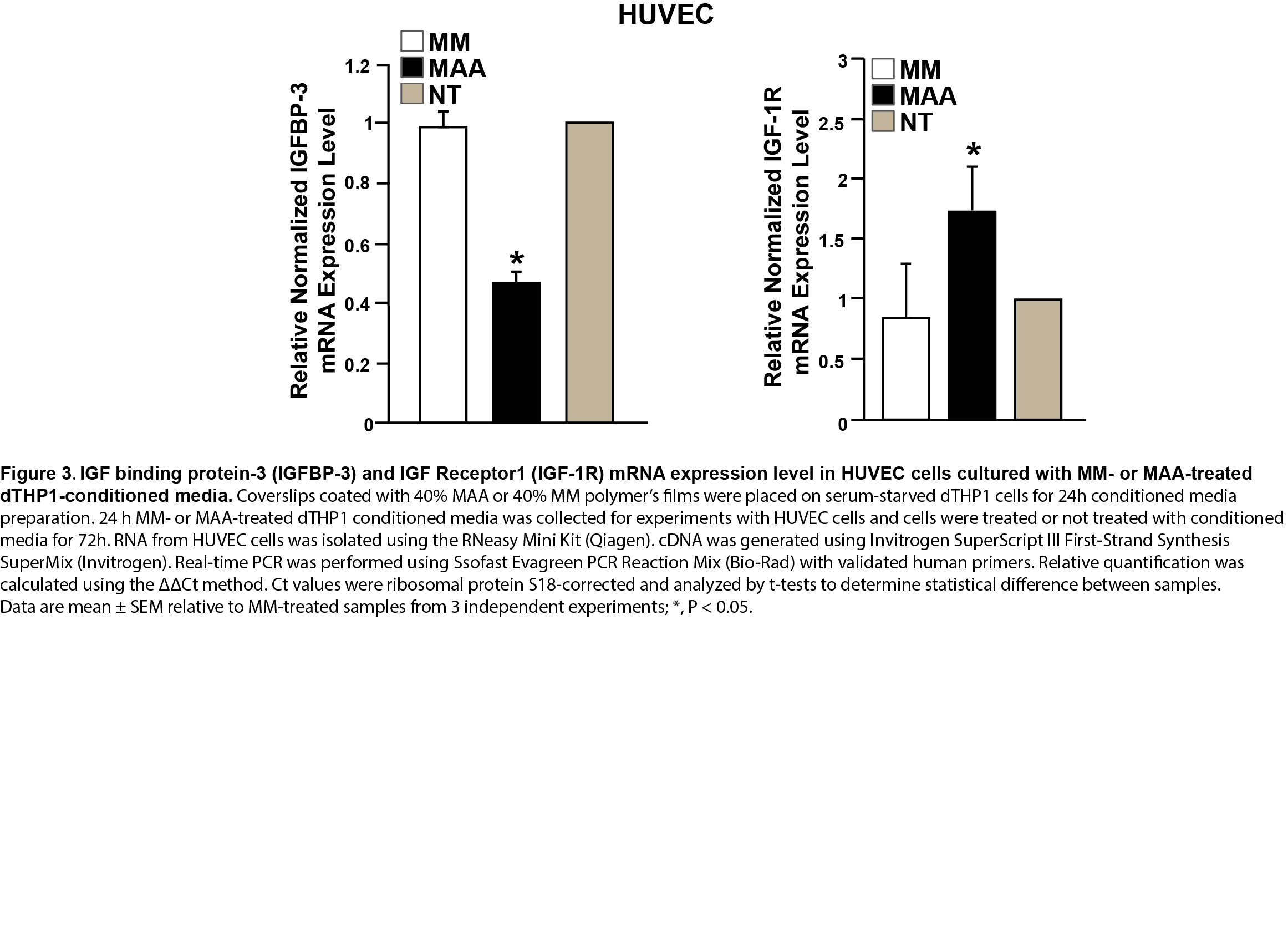
Conclusion: This data suggest that MAA-co-IDA stimulates an IGF-1 mediated macrophage response to the biomaterial, which in turn activates endothelial cells required for the process of vascularisation.
The next step for better understanding of the role of the IGF-1 dependent pathway, is to relate the observed findings in vitro to the angiogenic response in vivo.
Understanding of MAA stimulation mechanism can be used to advance the knowledge of this biomaterial and cell or tissue interaction, thereby facilitating the development of MAA polymer for new therapeutic applications, such as revascularization and tissue regeneration.
Ontario Research Fund
References:
[1] Eckhaus AA, Fish JS, Skarja G, Semple JL, Sefton MV. (2008). A preliminary stusy of the effect of poly(methacrylic acid-co-methyl methacrylate) beads on angiogenesis in rodent skin drafts and the quality of the panniculus carnosus. Plast Reconstr Surg 122 (5): 1361-1370.
[2] Lisovsky A, Chamberlain MD, Wells LA, Sefton MV. (2015). Cell Interactions with Vascular Regenerative MAA-Based Materials in the Context of Wound Healing. Adv Healthc Mater:1-13.
[3] Fitzpatrick LE, Chan JW, Sefton MV. (2011). On the mechanism of poly (methacrylic acid -co- methyl methacrylate)-induced angiogenesis: gene expression analysis of dTHP-1 cells. Biomaterials 32(34):8957-67.
[4] Bunn RC, Fowlkes JL. (2003). Insulin-like growth factor binding protein proteolysis. TREND in Endocrinology and Metabolism 14 (4): 176-181.
[5] Clemmons DR. (2007). Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discovery 6 (10): 821-833.