Introduction: Bupivacaine is one of the most widely used local anesthetic drugs containing the amino amide group. It is widely used in the treatment of pre/post-operative pain because of its rapid acting and relatively long-lasting anesthetic effect for up to several hours. However, for the patients experiencing chronic pain, a much longer period of drug exposure is needed at the site of interest. For this purpose, we prepared biodegradable microparticles loaded with bupivacaine and formulated them in fibrin glue. In this way, drug release could be more sustained via polymer matrices in both microparticles and fibrin glue. Fibrin glue herein could also localize the microparticles at the specific site of pain for a prolonged period, hence better local drug efficacy.
Materials and Methods: Bupivacaine-loaded microparticles were prepared by the O/W emulsion method. PLGA (200 mg; PLA:PGA=50:50; average M.W. = 48000) and bupivacaine hydrochloride (100 mg; ≥99%) were dissolved in 10 ml of methylene chloride. The resulting solution was added into the aqueous phase (200 ml of DI water with 2% PVA) followed by solvent evaporation at room temperature for 1 h. After filtration and drying, the drug loaded microparticles were obtained, which were then suspended in fibrin glue. In vitro drug release experiments were performed in pH 7.4 PBS at 37℃ for 35 days. In vivo experiments were conducted for 27 days using living rats, where the pain was induced with L5-spinal nerve ligation. At a local site of pain, we administered the formulation and the paw withdrawal latency was evaluated in response to thermal stimulation.
Results and Discussion: The bupivacaine loaded microparticles (B_MP) were successfully prepared with the O/W emulsion method, where the drug loading amount was 78.1 μg per mg microparticles. Figure 1 shows the in vitro drug release profiles of the microparticles formulated in fibrin glue. After a burst release of 38% on the first day, the drug was slowly released for more than a month. We also performed the in vivo experiments to evaluate the pain-relief efficacy of our formulation. As shown in Figure 2, the withdrawal latency decreased to less than 5 s with the pain-induced animal groups (control). The group treated with a bupivacaine solution (B_S) exhibited the increase in withdrawal latency for 1 day, which, however, decreased again afterwards due to rapid clearance of drug solute. Notably, for the B_MP in fibrin glue, the withdrawal latency was higher than those of sham and control, which was retained for 14 days. This result suggested that a local effect of bupivacaine could be prolonged and improved due to sustained drug release and microparticle localization with fibrin glue.
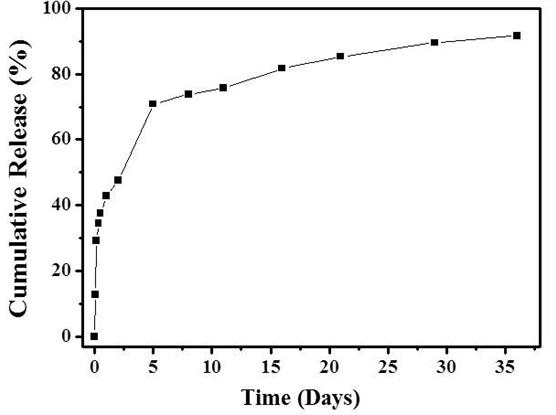
Figure 1. In vitro drug release profiles of the bupivacaine loaded microparticles in fibrin glue. The experiments were performed in pH 7.4 PBS at 37℃.
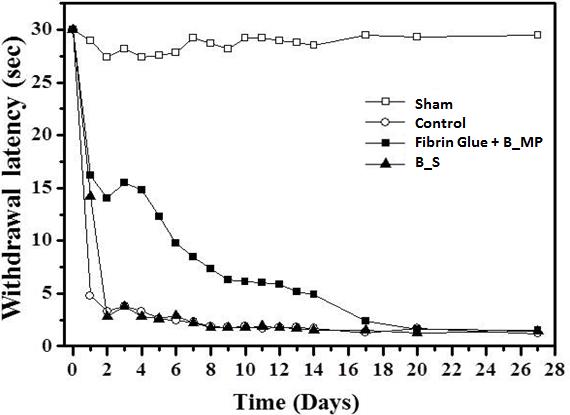
Figure 2. In vivo evaluation on paw withdrawal latency in response to thermal stimulation. (□; Sham group, ○; Control group, ■; Fibrin glue + bupivacaine loaded microparticles (B_MP), ▲; bupivacaine solution (B_S) group)
Conclusions: In this work, we suggest a composite formulation of anesthetic drug-loaded microparticles and fibrin glue as potential formulation for sustained drug delivery to the local site of pain. Our formulation revealed that the pain-relief efficacy could be prolonged for up to 14 days, where the enhancement of in vivo drug efficacy was evident as compared with the conventional treatment with an aqueous drug solute.
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (2015M3A9E2030129).; This research was supported by BK21 Plus Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant numbers 22A20130011025).
References:
[1] P. Chahar, K. C. Cummings III, Journal of Pain Research, 2012, (5), 257-264
[2] A. Shikanov, A. J. Domb, C. F. Weiniger, Journal of Controlled Release, 2007, (117), 97-103
[3] J. Curley, J. Castillo, J. Hotz, M. Uezono, S. Hernandez, J. Lim, J. Tigner, M. Chasin, R. Langer, C. Berde, Anesthesiology, 1996, (84), 1401-1410
[4] D. H. Sierra, Journal of biomaterials apprlications, 1993, (7), 309-352