Statement of Purpose: Alcohol use disorders continue to disrupt millions of lives, resulting in a significant economic burden of over $224 billion annually in the US[1]. Pharmacotherapy continues to support the greatest clinical outcomes but there are limited FDA-approved treatments, with patient compliance being the largest barrier for clinical success. An alternative approach involves local, sustained controlled release of an existing therapeutic with a proven clinical benefit (disulfiram) to maximize patient compliance and improve clinical outcomes[2]. Therefore, the present study examines the ability for sustained release of disulfiram from an injectable, amphiphilic, absorbable, depot-forming drug delivery system (DDS) – Viscoprene®.
Methods: Viscoprene® 6519 was polymerized as described previously[3] to form a polyether ester urethane containing 65% d,l-lactide, 19% polyethylene glycol, and 16% glycolide interlinked with an aliphatic di-isocyanate with an initial molecular weight of 12,800 Daltons. This polymer was used to prepare the injectable DDS using 2:1 Viscoprene™ 6519:PEG 400 (Sigma Aldrich) and drug loaded by dissolving 5% and 8.5% disulfiram (Sigma Aldrich). In vitro degradation was performed using neat polymer according to ASTM F1635-11 using 100 mM phosphate buffered solution, pH=7.4 at 37°C with samples analyzed for mass loss profile and molecular weight through the study duration. Molecular weight was measured using a PerkinElmer GPC with THF mobile phase. Throughout the degradation time, cytotoxicity was evaluated using an MTT assay as well as visual microscopy with positive and negative controls. Disulfiram release was analyzed using a depot release methodology at 37°C. Eluents were collected and analyzed using a Waters HPLC equipped with PDA detector, using ACN/water mobile phase and an isocratic method.
Results and Discussion: Disulfiram-loaded Viscoprene® DDS was initially tested for suitability with both loading concentrations through hand injection trials using an 18-gauge needle. The DDS readily formed a depot in buffer while maintaining molecular dispersion of drug. Figure I provides a summary of MTT, degradation study, and drug release data through the in vitro study period. Degradation of the Viscoprene® DDS depot exhibited molecular weight loss to approximately 3,000 Daltons, with delayed mass loss primarily by dissolution of lower molecular weight degradation products, consistent with a polyester-based hydrogel system. Results of the MTT assay and visual microscopy indicate the material has little to no cytotoxic effect through the degradation study period, predicting safety particularly of the aliphatic isocyanate chain extender. Disulfiram release analysis indicated less than 5% loss due to burst release and a consistent release profile through the study duration. Interestingly, the 5% loaded DDS released disulfiram at a faster rate than the DDS loaded with 8.5% drug. Cytotoxicity and degradation study data consist of triplicate samples.
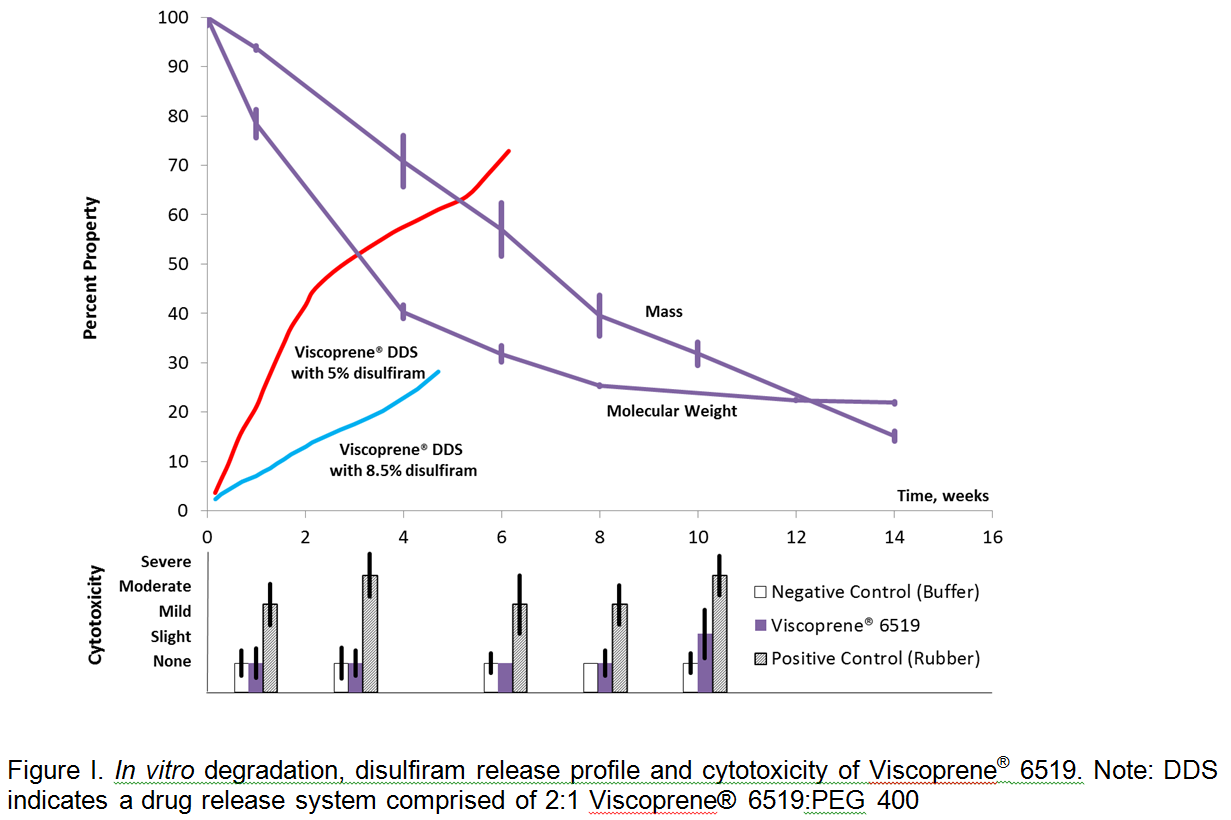
Conclusion: The Viscoprene® DDS was designed to provide controlled and sustained delivery of active agents over a clinically relevant duration. The present study confirmed the capability of forming a degradable, drug-eluting depot, delivering disulfiram over an extended period of time. Preliminary safety data indicates the viability of the system, and the consistent release over more than four weeks with minimal burst effect predicts the potential of this system for use as an improved treatment for alcohol use disorders by replacing the need for daily administration of oral tablets.
References:
[1] NIH. Alcohol Facts and Statistics. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics. March 2015.
[2] Jorgensen, C.H., Pedersen, B., Tonnessen, H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 35(10):1749-58. 2011.
[3] Shalaby, S.W. Bioactive polymeric liquid formulations of absorbable, segmented aliphatic polyurethane composition. US Patent 8.952,075 B2. 2015.