Introduction: The formation of a proper soft-tissue sealing is essential for the performance of long-term percutaneous (through the skin) implants and is currently one of the main challenges in the implant industry aimed at e.g. Bone-Anchored-Hearing-Devices and Percutaneous Osseointegrated Prostheses[1]. The risks of infection for medical devices penetrating the skin is, however, still a concern and limits the use of such devices[2]. The main issue is related to the skin-implant interface where the barrier-forming epidermal layer of the skin does not from a stable interface with the implant[3].
Testing this interface is not possible in-vitro using standard cell culture, as it does not represent the complexity of the skin. On the other hand animal models elicit the full cutaneous response, but are expensive, not suitable for a screening approach and have some ethical concerns.
We therefore aim at developing a model that can be used to get further insight into the creation of the skin-biomaterial interface[4] and as an advanced prescreening to in vivo models for suitable implant functionalization to increase epidermal adhesion and test barrier function.
Materials and Methods: Skin is obtained from skin reduction surgery and shortly after surgery the top layer (400µm) of the skin is removed using a dermatome. An implant is inserted through the skin and placed in a 12 well plate. Modified serum free Williams E media[5] is added and the skin is placed at the air-liquid interface using an alginate hydrogel as a scaffold. The samples are incubated at 37 degrees, 5% CO2 for up to eight days. Samples are fixated in ethanol and embedded in PMMA. The samples are subsequently cut on a Leiden saw and stained using hematoxylin and eosin (H&E). We tested this model using ø1mm rods made from grade 5 titanium and silicone.
Results and Discussion: After culture the skin cells were viable as examined by histology and the skin maintained its structure with a clearly separated dermal and epidermal layer. The epidermis still maintained its distinct layers and migration of the epidermis on the dermal layer was observed, showing that the model can be used to understand epidermal down growth around an implant. So far we have used the system to test grade 5 titanium and silicone with respect to tissue adherence. Next step will be to include surface modifications.
The use of both hard and soft implants, allows for testing of modifications relevant to both bone-anchored implants and catheters

Figure 1: An alginate hydrogel serves as scaffold for the 400um thick layer of skin, a grade 5 titanium implant penetrates both.
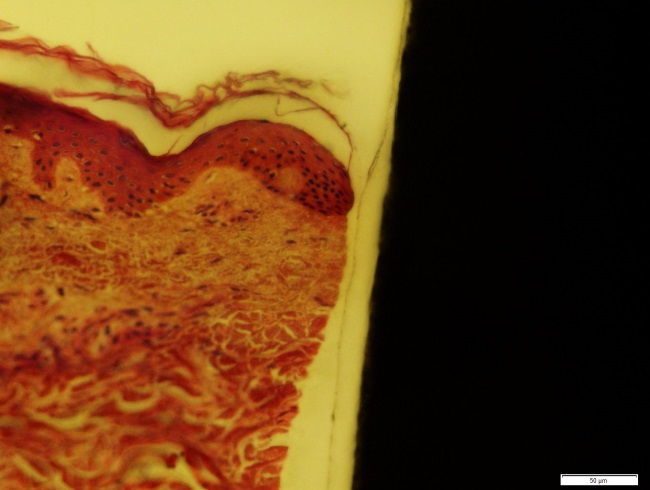
Figure 2: A section showing the titanium implant on the right and the skin on the left, this section is taken right after insertion so no adhesion between implant and skin is observed.
Conclusion: We have developed an ex vivo model where the interface between a metal implant and skin can be investigated in-vitro for the first time. This model allows for investigation of the influence of surface modification of the implant on the skin-biomaterial interface. The model is suitable as a platform for screening approaches and has no ethical concerns.
References:
[1] Dun CAJ, Faber HT, de Wolf MJF, Mylanus EAM, Cremers CWRJ, Hol MKS. Assessment of More Than 1,000 Implanted Percutaneous Bone Conduction Devices. Otol Neurotol. 2012;33:192-198
[2] Knowles NG, Miyashita Y, Usui ML, et al. A model for studying epithelial attachment and morphology at the interface between skin and percutaneous devices. J Biomed Mater Res - Part A. 2005;74:482-488.
[3] Affeld K, Grosshauser J, Goubergrits L, Kertzscher U. Percutaneous devices: a review of applications, problems and possible solutions. Expert Rev Med Devices. 2012;9:389-399.
[4] Fleckman P, Olerud JE. Models for the histologic study of the skin interface with percutaneous biomaterials. Biomed Mater. 2008;3:034006. Fleckman P, Olerud JE. Models for the histologic study of the skin interface with percutaneous biomaterials. Biomed Mater. 2008;3:034006.
[5] Lu Z, Hasse S, Bodo E, Rose C, Funk W, Paus R. Towards the development of a simplified long-term organ culture method for human scalp skin and its appendages under serum-free conditions. Exp Dermatol. 2007;16(1):37-44.