Scaffold-based tissue engineering approaches often rely on cells to remodel the scaffold into native tissues [1][2]. However, the resulting inflammatory response or insufficient oxygen or nutrient delivery can be problematic after implantation. Cell-based strategies have been suggested as an alternative approach [3][4], but low survival efficiency and difficulty in localization and geometrical organization of injected cells reduced their therapeutic effects. In this study, the structural support of degradable polymers with a targeted cellular strategy using cell sheet engineering technology was used to improve survival efficacy and control the structural organization of cells. In this system, we used two types of enzymatically degradable hydrogels as a sacrificial substrate to harvest cell sheets without damaging extracellular matrix proteins (ECM). Moreover, the substrate was patterned to control the phenotype of the vascular smooth muscle cell sheet and to structurally mimic the native vascular wall of medial layer.
Materials and Methods: Cellulose (CMC), alginate (Al), 1-Hydroxybenzotriazole hydrate, tyramine hydrochloride, MES, EDC and NHS were purchased from Sigma Aldrich. Tyramine-conjugated hydrogel (CMC-ty, Al-ty) was crosslinked by horseradish peroxidase/H2O2, and degraded by specific enzymatic treatment (cellulase, alginate lyase) within medium, allowing intact cell sheets to be harvested without damaging the cells or ECM. Vascular Smooth Muscle Cells (VSMCs) were cultured on these substrates to form cell sheets (Fig 1). Cellular morphology and cytoskeletal structure were observed by F-actin staining. Phenotypic gene expression was analyzed by PCR and tensile properties of cell sheet were measured by a custom uniaxial tensile tester [5].
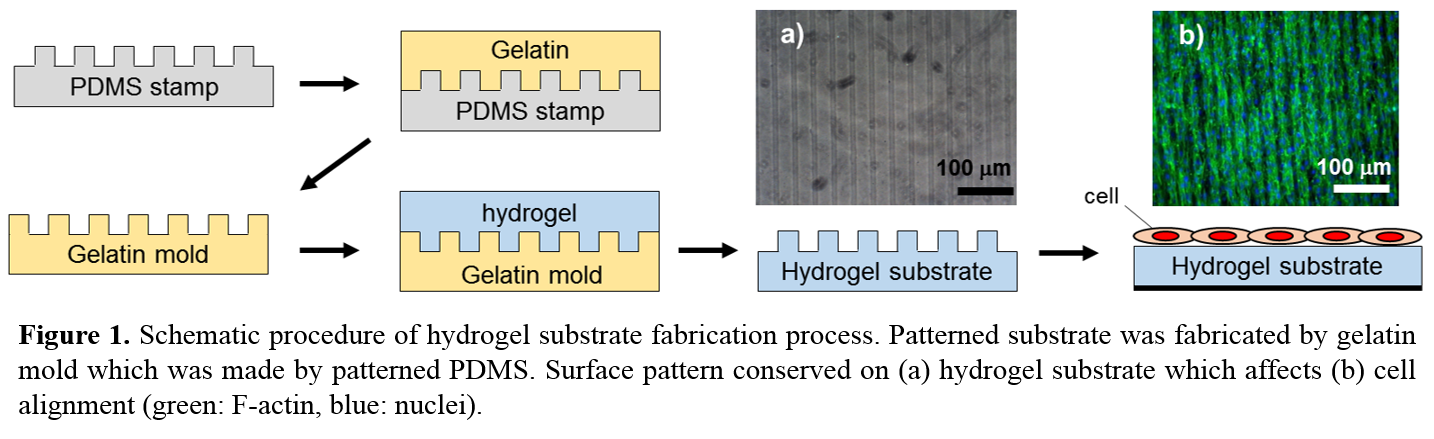
Results and Discussion: The cell alignment maintained during proliferation, and once the cells were confluent, we confirmed that the cytoskeletal integrity of the cell sheet was maintained with high cellular viability after cell sheets transfer to another substrate. The patterned cell sheet showed statistically increased/decreased contractile/synthetic gene expression profiles, respectively, relative to non-pattern cell sheets (Fig 2). Patterns of each layer of multi-cell sheet construct were visualized via confocal microscopy. For further functional characterization, tensile properties of cell sheets were determined after harvesting from patterned/non-patterned substrates.
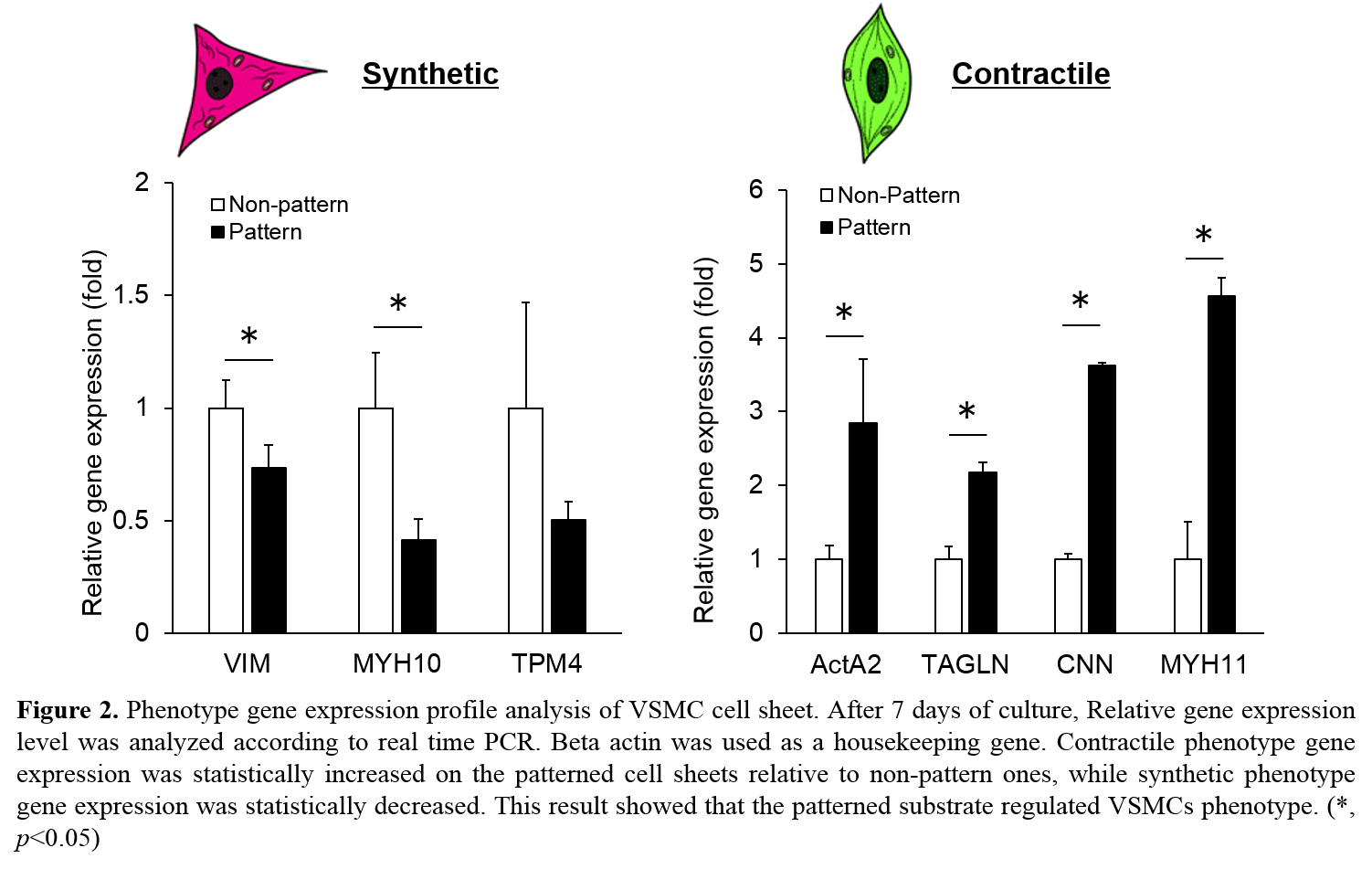
Conclusions: In this work, we validated a cell sheet harvesting system using sacrificial substrates with structural organization that retained patterns and cytoskeletal structure of cells after cell sheet harvest and controlled the phenotype which was confirmed by gene expression. With the use of biochemically and mechanically characterized cell sheets, this system provides a tool for scaffold-free patches with intact cellular structure and biocompatibility that can potentially be applicable for the creation of complex tissue that mimics the properties of vasculature.
Nae Gyune Rim is a Howard Hughes Medical Institute International Student Research fellow.; This work was partially supported by The Hartwell Foundation.
References:
[1] Haraguchi, Y., et al., Regenerative therapies using cell sheet-based tissue engineering for cardiac disease. Cardiology research and practice, 2011. 2011: p. 845170.
[2] Matsuura, K., et al., Cell sheet transplantation for heart tissue repair. Journal of controlled release : official journal of the Controlled Release Society, 2013. 169(3): p. 336-40.
[3] Terrovitis, J., et al., Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. Journal of the American College of Cardiology, 2009. 54(17): p. 1619-26.
[4] Hofmann, M., et al., Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation, 2005. 111(17): p. 2198-2202.
[5] Isenberg, B.C., et al., Micropatterned cell sheets with defined cell and extracellular matrix orientation exhibit anisotropic mechanical properties. J Biomech, 2012. 45(5): p. 756-61.