Introduction: Intracellular protein delivery have been received a great attention in recent years for various clinical applications. However, they are often exhibited several inherent problems such as difficulty to enter in plasma membrane and entrapping in lysosomes. Previously, we have developed a new methodology called freeze concentration for enhanced cytoplasmic protein delivery[1]. Freeze concentration is a physicochemical phenomenon where ice crystallizes from water, leading to increase in concentration of solutes in the remaining unfrozen solution. This results in phase separation between ice and concentrated solutes across the system. This strategy was strongly facilitated the enhanced adsorption and internalization due to increase in concentration across peripheral cells. However, mechanism of internalization was unclear. Herein, we report an efficient endosomal escape and endocytic uptake after protein internalization through freeze concentration.
Materials and Methods: We prepared two types of liposomes, one is made of DOPC/DOPE liposome and other is polyampholyte modified liposomes. Briefly, DOPC/ DOPE solution was prepared by lipid film hydration method with lysozyme protein containing solutions with or without polyampholyte and subsequently extruded. One million L929 cells were cryopreserved by unmodified or polyampholyte modified liposomes encapsulating protein were frozen at -80 ̊C in the presence of cryoprotectant. The solutions were thawed and washed with PBS (-) 3 times. Thawed cells were seeded to glass bottom dish for internalization. Adsorption and internalization of protein were investigated by flow cytometry and confocal laser scanning microscope.
Results and Discussions: Flow cytometry analysis shows a strong green fluorescence signals from protein were observed after protein adsorption by facile freeze thaw method rather than unfrozen.
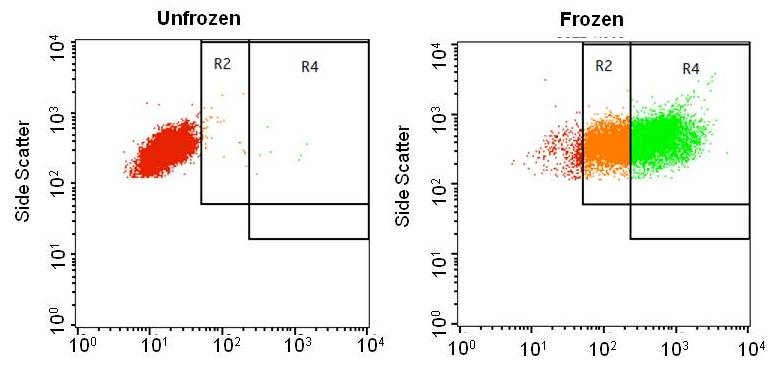
The freeze concentration factor might concentrate the molecules bound to the surface of the cells and enhances the protein adsorption. Furthermore, the endocytic uptake of protein/liposome complex was investigated by inhibition studies[2]. These studies revealed that the unmodified and polyampholyte modified liposomes adopt distinct pathway of protein internalization. Moreover, our results demonstrated the new polyampholyte modified liposome showed endosomal escape property and introduction of lysozyme protein to the cytoplasm by using freeze concentration approach.
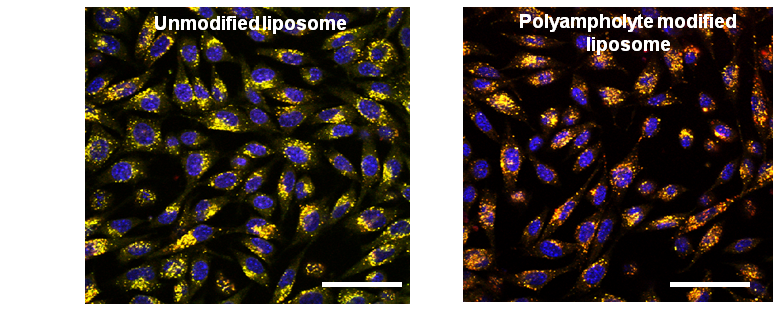
Polyampholyte modified liposomes could adsorb on to the cells and destabilize the endosomal membrane which led to release of protein. These results found an enhanced in vitro endosomal efficacy with low toxicity.
Conclusions: This study indicated positive results of intracellular protein delivery by using freeze concentration These findings will provide a mechanistic overview of intracellular behavior of protein. Polyampholyte modified liposomes carriers can be beneficial for cancer immunotherapy.
References:
[1] S. Ahmed, F. Hayashi, T. Nagashima and K. Matsumura, “Protein cytoplasmic delivery using polyampholyte nanoparticles and freeze concentration,” Biomaterials. Vol. 35, Aug. 2014.
[2] T. Akagi, F. Shima and M. Akashi, “Intracellular degradation and distribution of protein encapsulated amphiphilic poly(amino acid) nanoparticles,” Biomaterials. Vol. 32, Jul. 2011.