Introduction: In developing a clinically-translatable platform for adipose tissue replacement in reconstructive surgery, our lab utilizes decellularized adipose tissue (DAT), a bioactive scaffold with the capacity to stimulate the adipogenic differentiation of adipose-derived stem cells (ASCs), with or without exogenous differentiation factors, by providing a unique adipo-conductive and adipo-inductive ECM microenvironment[1]. DAT derived scaffolds have also been shown to stimulate angiogenesis in vivo[2]. In this study, we present recent progress towards elucidating the DAT composition to gain a deeper understanding of the bioactive properties, assess donor variability and optimize the regenerative potential of these highly-promising matrices.
Materials and Methods: Adipose tissue was acquired from female patients undergoing elective surgery at the University Hospital in London, Canada, with approval from Western University (HREB 105426). The tissue was decellularized using an established 5-day protocol[1] or a refined 3-day protocol, which was developed to reduce processing time and costs, as a step towards scale-up for commercial production. Both DAT protocols were validated to remove cellular components and assessed for biocompatibility in an immunocompetent rat model over 16 weeks. The retention of structural and cell adhesion proteins native to adipose tissue and distribution of growth factors associated with regeneration was evaluated by IHC. To gain a broader compositional understanding, DAT was enzymatically digested and peptides fractionated before analysis using an Orbitrap (Q-Exactive) mass spectrometer (LC-MS/MS)[3].
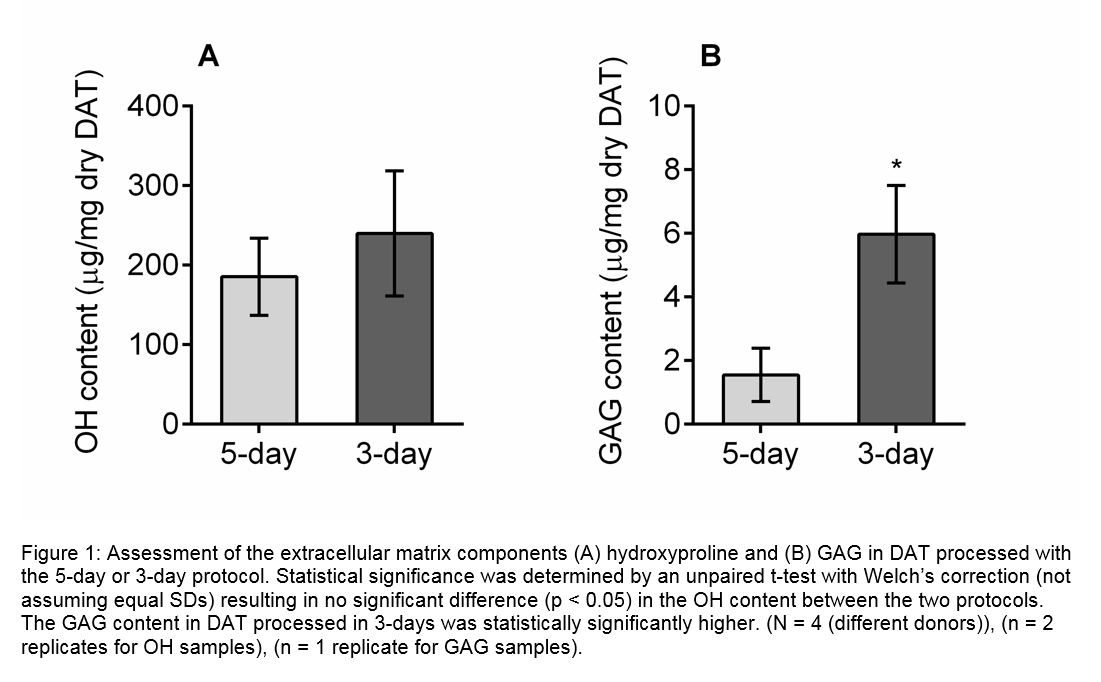
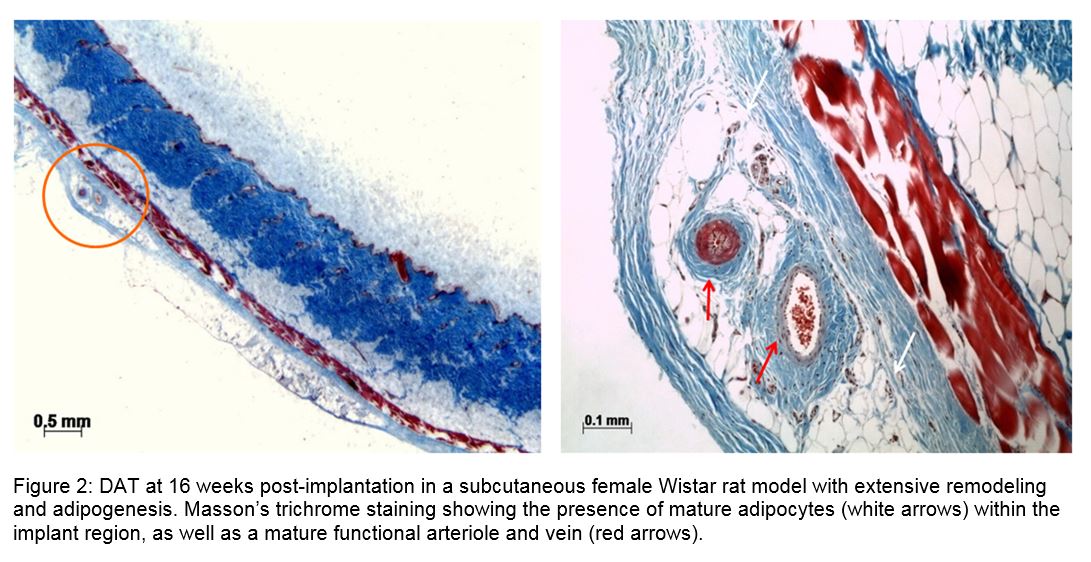
Results and Discussion: IHC demonstrated the retention of collagen I and IV, laminin (LN), fibronectin (FN), VEGF-A and adiponectin in the DAT. Increased levels of protein were extracted from the refined DAT protocol, which was also found to preserve higher GAG content (Fig. 1). Both DAT protocols stimulated host soft tissue regeneration with vascularization when implanted in vivo (Fig. 2). Mass spec analysis identified 804 unique proteins in the soluble fraction of the scaffold. Structural proteins, including 21 different collagens, elastin, and fibrilin-1 were identified in the DAT. Glycoproteins (FN, LN, vitronectin) and proteoglycans (perlecan, versican) were also identified, which have been associated with cell functions including adhesion, migration, proliferation and growth factor activity. The DAT scaffold also contains multiple growth factors (FGF-2, FGF-5, TGF-βI), cytokines (adiponectin, angiopoietin-related protein 2, betatrophin) and matricellular proteins (dermatopontin, galectin-1-3, periostin), many of which are affiliated with wound healing and tissue regeneration.
Conclusion: Overall, we have developed a highly promising and clinically translatable platform which has been optimized and characterized for commercialization as a biomaterial for adipose tissue replacement.
Funding for this study was provided by the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Canadian Institutes of Health Research (CIHR). The authors would like to thank Dr. B. Evans and Dr. A. Grant for their clinical collaborations.
References:
[1] L.E. Flynn, “The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells,” Biomaterials. Vol. 31, June 2010
[2] T. Han, S. Toutounji, B. Amsden, et al, “Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds,” Biomaterials. Vol 72, August 2015
[3] C. Hughes, L. Radan, W. Chang et al, “Mass spectrometry-based proteomic analysis of the matrix microenvironment in pluripotent stem cell culture,” Molecular & Cellular Proteomics. Vol. 11, September 2012