Introduction: The adsorption of a complex multi-component layer of proteins is the first significant event when a biomaterial comes in contact with blood. Apolipoprotein AI (apo AI), the main protein of high density lipoprotein (HDL), has been identified as a major component of the protein layer formed on surfaces in contact with blood both in vitro and clinically [1], suggesting that apo AI and HDL, may play an important, though heretofore overlooked, role in blood-material interactions. Whether such lipoprotein interfacial interactions are beneficial or detrimental to blood compatibility is unknown. In previous work we determined the quantities of apo AI bound to a clinically relevant polyurethane, both from buffer and human plasma [2]. We also quantified the adsorption of apolipoproteins AII and B, and of the lipoproteins HDL, LDL and VLDL, from buffer [3].
The work reported here provides quantitative data on the adsorption of these apolipoproteins and lipoproteins from single component and binary solutions in buffer, and from the more clinically relevant medium, plasma, to a commercially available biomedical grade polyurethane, Tecothane®, and to Tecothane® containing a polyethylene oxide (PEO)-based additive, expected to provide resistance to adsorption.
Materials and Methods: Tecothane® (TT-1095A) was used as a model surface. Tecothane blended with 10% of a polyethylene oxide-based triblock copolymer (blended material referred to as “PEO”), which has been shown to be strongly protein resistant[4], was used to investigate whether the adsorption of lipoprotein particles, as well as molecular apolipoproteins is inhibited on this surface. Films of thickness ~0.5 mm were prepared, and 6 mm diameter disks punched from the films for adsorption experiments.
Purified apolipoproteins (apo AI, apo AII, apo B) and lipoproteins (HDL, LDL, VLDL) were obtained commercially and radiolabelled with I-125 or I-131. Adsorption experiments were carried out by incubating (2 h) the surfaces in single component and binary solutions in buffer, and in citrated human plasma containing the radiolabelled proteins as tracers, and determining the quantities bound to the surfaces.
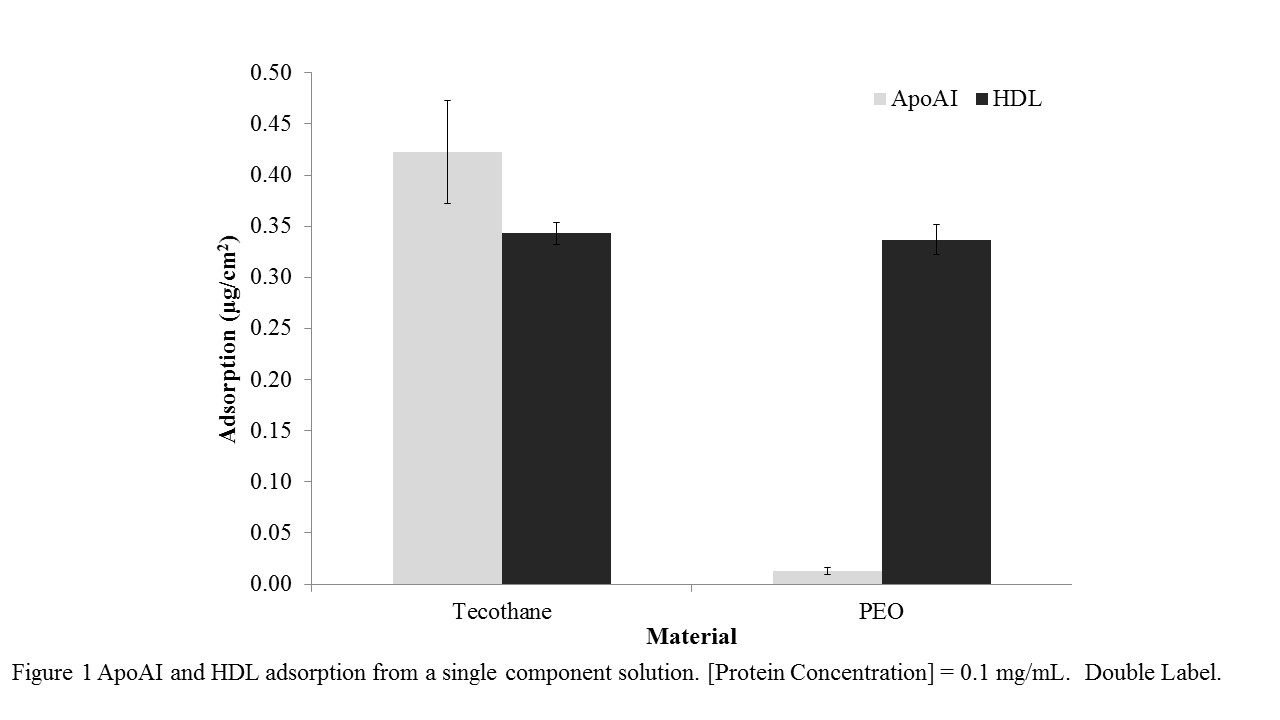
Results and Discussion: Adsorption of both apo AI and HDL to Tecothane from single component solutions (0.1 mg/mL) was significant and quantitatively similar (Figure 1). Adsorption of apo AI to the PEO surface was reduced by >95% compared to Tecothane (Figure 1). However the PEO surface was not resistant to HDL. Experiments using binary mixtures of apo AI (labelled with I-125) and HDL (labelled with I-131), concentrations 0.1 mg/mL, showed that on Tecothane, adsorption was again significant but HDL was preferred over apo AI. On the PEO surface apo AI was almost completely suppressed, but HDL was adsorbed at a level similar to that from a single component HDL solution.
In plasma experiments it was found that significant quantities of the apolipoproteins and lipoproteins were adsorbed to Tecothane. On the PEO surface in plasma, adsorption of the apos was minimal, but the lipoproteins adsorbed significantly to the PEO. The lack of resistance of the PEO surface to the lipoproteins was thus a consistent finding across all experiments, and may reflect the more hydrophobic character of the lipoprotein surfaces compared to the “free” apolipoproteins. It may also suggest a limitation on the effectiveness of PEO as a “passivating” surface modifier for blood contacting applications.
Conclusions: Apolipoproteins and lipoproteins were shown to adsorb from buffer and plasma to a medical grade polyurethane. Somewhat surprisingly, lipoproteins (but not apolipoproteins) were also shown to bind to a PEO surface shown previously to be strongly protein resistant[3][4]. In a broader context, apo AI- HDL are known to have beneficial effects including cardioprotective, anti-inflammatory, antibacterial, and antiviral; metal surfaces designed to bind HDL selectively in blood contact were shown to be antithrombotic in vitro[5]. Thus control of the interactions of these and other lipoproteins at the blood-material interface may be a useful approach in the development of thromboresistant surfaces.
References:
[1] R.M. Cornelius et al, Biomaterials, 2002, 23, 3583.
[2] R.M. Cornelius et al, J Biomed Mater Res, 2011, 99A, 109.
[3] R.M. Cornelius et al, SFB Annual Meeting, 2014.
[4] J. Tan and J.L. Brash. J Biomed Mater Res, 2009, 90A, 196.
[5] A.C. Strang et al, PloS One, 2015, 10(3): e0122836.