Introduction: Injectable hyaluronic acid (HA) hydrogels have been increasingly applied in tissue engineering (TE) envisioning minimal invasive approaches. However, traditional HA hydrogels lack structural integrity that makes them less competitive in strategies where good mechanical properties are required. Here we propose the use of cellulose nanocrystals (CNCs), the nature “carbon nanotubes”, as nanofillers and crosslinkers in a fully biobased strategy for the production of reinforced HA nanocomposite hydrogels[1]. Due to their distinct mechanical properties, biocompatibility and excellent aqueous colloidal stability, CNCs are being increasingly considered in hydrogel development targeting biomedical applications[2]. We hypothesise that besides structural reinforcement, in TE strategies the CNC’s surface SO3- groups may also potentially act as semisynthetic mimicry of ECM sulfated glycosaminoglycans, which are known to induce and control specific cell functions on the cellular microenvironment through interactions with soluble biomolecules, e.g. proteins, growth factors (GFs)[3].
Materials and Methods: In situ crosslinkable and injectable hydrogels were prepared based on hydrazone coupling of adipic acid dihydrazide-modified HA (ADH-HA) and aldehyde-modified HA (a-HA), reinforced with aldehyde-modified CNCs (a-CNCs) (Figure 1). The hydrogel precursors were fully characterized by several spectroscopic, chromatographic, and imaging techniques, and the hydrogels were characterized in terms of internal morphology, mechanical properties, swelling and degradation behaviour in the presence of hyaluronidase. The biological performance of the developed nanocomposites was assessed towards human adipose derived stem cells (hASCs).
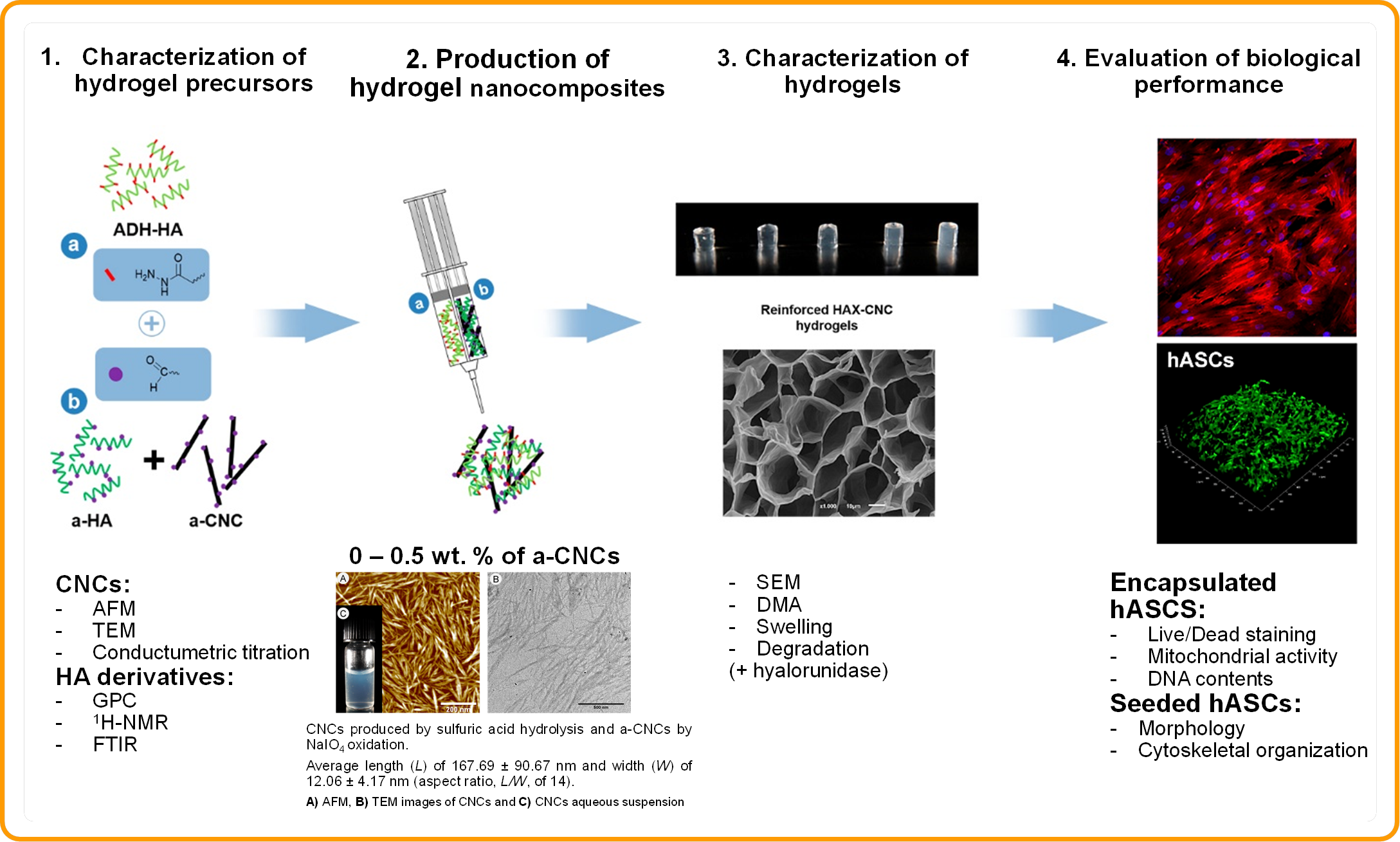
Figure 1. Schematic representation of the nanocomposite hydrogels production, characterization and biological performance evaluation. Adapted with permission from ref. [1]. Copyright 2015 American Chemical Society.
Results and Discussion: The incorporation of a-CNCs in the hydrogel’s network had a remarkable impact over the physical and biological performance of the injectable biomaterial. Nanocomposite hydrogels showed improved microstructure and mechanical properties (increased E’ up to 2.7-fold compared to unfilled hydrogels), lower equilibrium swelling ratios and higher resistance to bulk hyaluronidase degradation. HA-CNCs exhibited preferential cell supportive properties in in vitro culture conditions, in both surface cell seeding and cell encapsulation tests. Particularly, hASCs encapsulated in HA-CNCs hydrogels demonstrated ability to spread within the volume of gels and exhibited pronounced proliferative activity. This impact over cell’s behaviour is correlated with the higher structural integrity of the hydrogel matrix and potential interaction of soluble microenvironmental cues with the CNC’s surface sulphate groups.
Conclusions: The proposed strategy demonstrated to be a valuable approach for fine tuning the structural, biomechanical and biochemical properties of injectable HA hydrogels. The combined effects of enhanced stability and mechanical properties with the incorporation of mimetic ECM biochemical cues in HA-CNCs hydrogels, proved to positively impact their biological performance for TE applications. Considering the promising outcomes, we are currently exploring the potential of the developed system when combined with discrete GFs or the GFs pool from platelet lysates in specific TE strategies.
The authors acknowledge the financial support from the Project RL1 - ABMR - NORTE-01-0124-FEDER-000016 cofinanced by North Portugal Regional Operational Programme (ON.2 – O Novo Norte), under the National Strategic Reference Framework (NSRF), through the European Regional Development Fund (ERDF)
References:
[1] Domingues RMA, Silva M, Gershovich P, Betta S, Babo P, Caridade SG, et al. Development of Injectable Hyaluronic Acid/Cellulose Nanocrystals Bionanocomposite Hydrogels for Tissue Engineering Applications. Bioconjugate Chemistry. 2015;26:1571–81.
[2] Domingues RMA, Gomes ME, Reis RL. The Potential of Cellulose Nanocrystals in Tissue Engineering Strategies. Biomacromolecules. 2014;15:2327-46.
[3] Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530-41.