Introduction: Fibroblast-seeded collagen gel which constructs a 3D bio-artificial extracellular matrix (ECM) has been widely used in wound healing, tissue engineering and regenerative medicine. Previous studies using fibroblast-populated collagen based assay to measure cell contractility were limited to examine the radius changes of gel and hence accurate determination of cell contraction force was difficult to achieve[1],[2]. Up to now, little has been known about how the stiffness of collagen matrix influences on cell contraction force. We have recently developed a novel nano-indentation device to measure the gel elasticity and in combination with a mathematical model, the cell contraction force can be determined accurately based on the measured gel thickness and area[3]. In this study, we apply the new technique to investigate the effect of gel stiffness on cell contraction force in a quantitative manner.
Materials and Methods: Human Aortic Adventitial Fibroblast (HAoAF) at Passage 7-10 were used to seed into collagen gel matrix. Type I collagen diluted with sterile water, resulting the final concentration of 1.5, 2.0 and 2.5 mg/ml, was mixed with 2 mM L-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin and 5% FCS. The collagen matrix suspension was transferred into 35 mm Petri dishes (1.2 ml/dish) and incubated for 20 minutes to polymerise. After incubation overnight, the culture medium was replaced with fresh DMEM containing 5% FCS. Some dishes were then stimulated with the agonist histamine (100 µM) to elicit cell contraction.
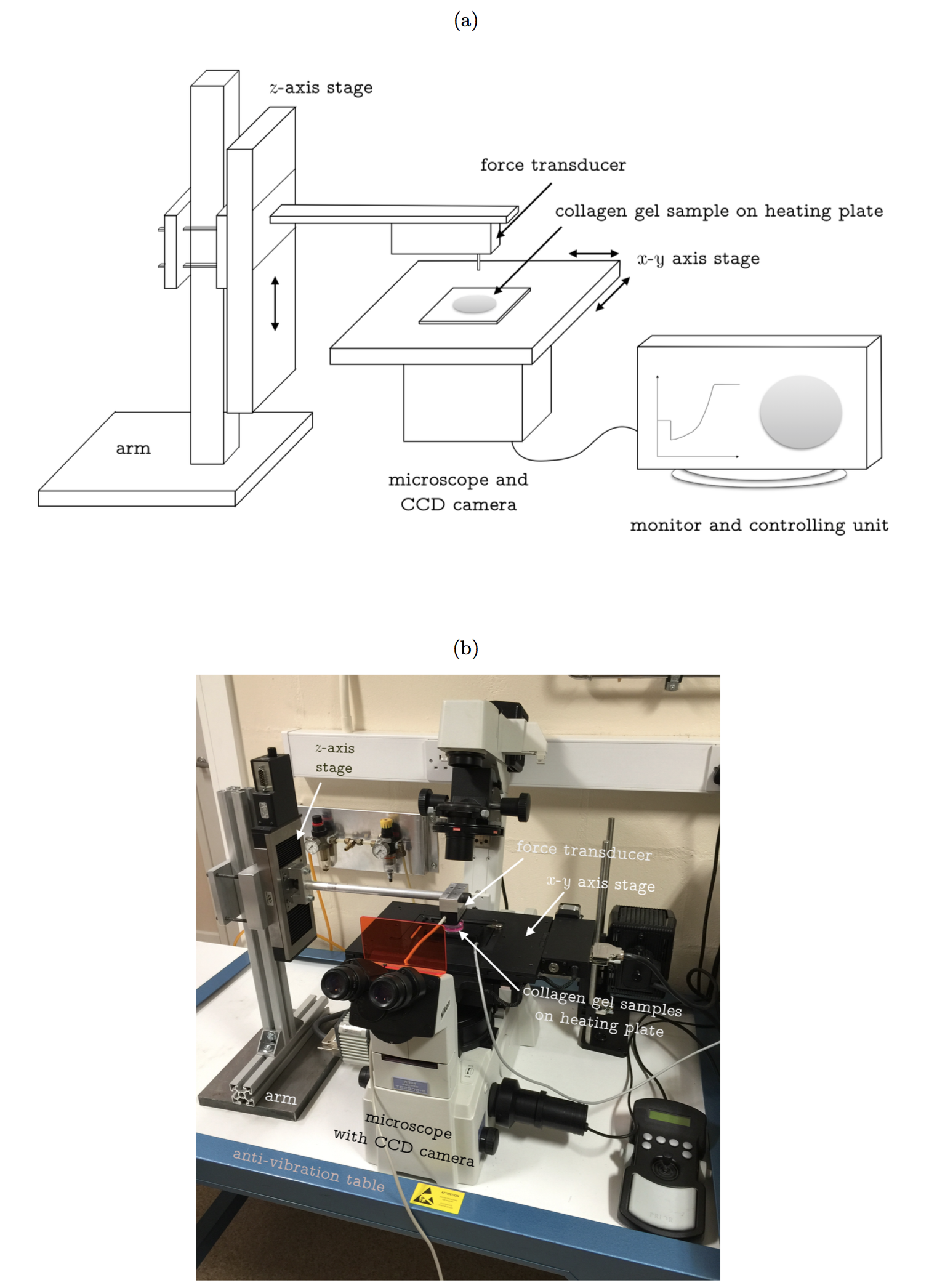
A depth-sensing indentation device (shown as Figure 1) is used to measure thickness and Young’s modulus of the cell-embedded collagen gel. The system has ultimate force and displacement resolutions of 10 nN and 100 nm, respectively. The gel indentation was performed at a controlled speed of 40 µm/sec to generate Force-displacement (F-d) curve. The first 30% of F-d curve was extracted to determine Young’s modulus (E) by fitting with a non-linear strain dependent elasticity model. The cell contraction force (F) can be calculated by the following equation.
F = πE(h0+h1)(r0-r1)
where h and r with subscript 0 and 1 represent the thickness and radius of disk shaped collagen gel at beginning and end of contraction.
Results and Discussion: Figure 2(a) shows overall contraction force of cells decreased as the collagen concentration increases. Meanwhile, the contraction forces were nearly doubled when cells were treated with histamine (100 µM). Figure 2(b) demonstrates a linear decrease in cell contraction force as the gel concentration increases. Figure 2(c) shows the Young’s modulus of gels increases in a higher collagen concentration. The results suggest that the concentration of collagen matrix regulates the fibroblast contraction force in response to the stimulation of agonist Histamine. The findings also show cells response differently to the stiffness change of collagen matrix. As a result, the Young’s modulus of gel matrix should be considered as a key parameter to assess the cell contraction force.
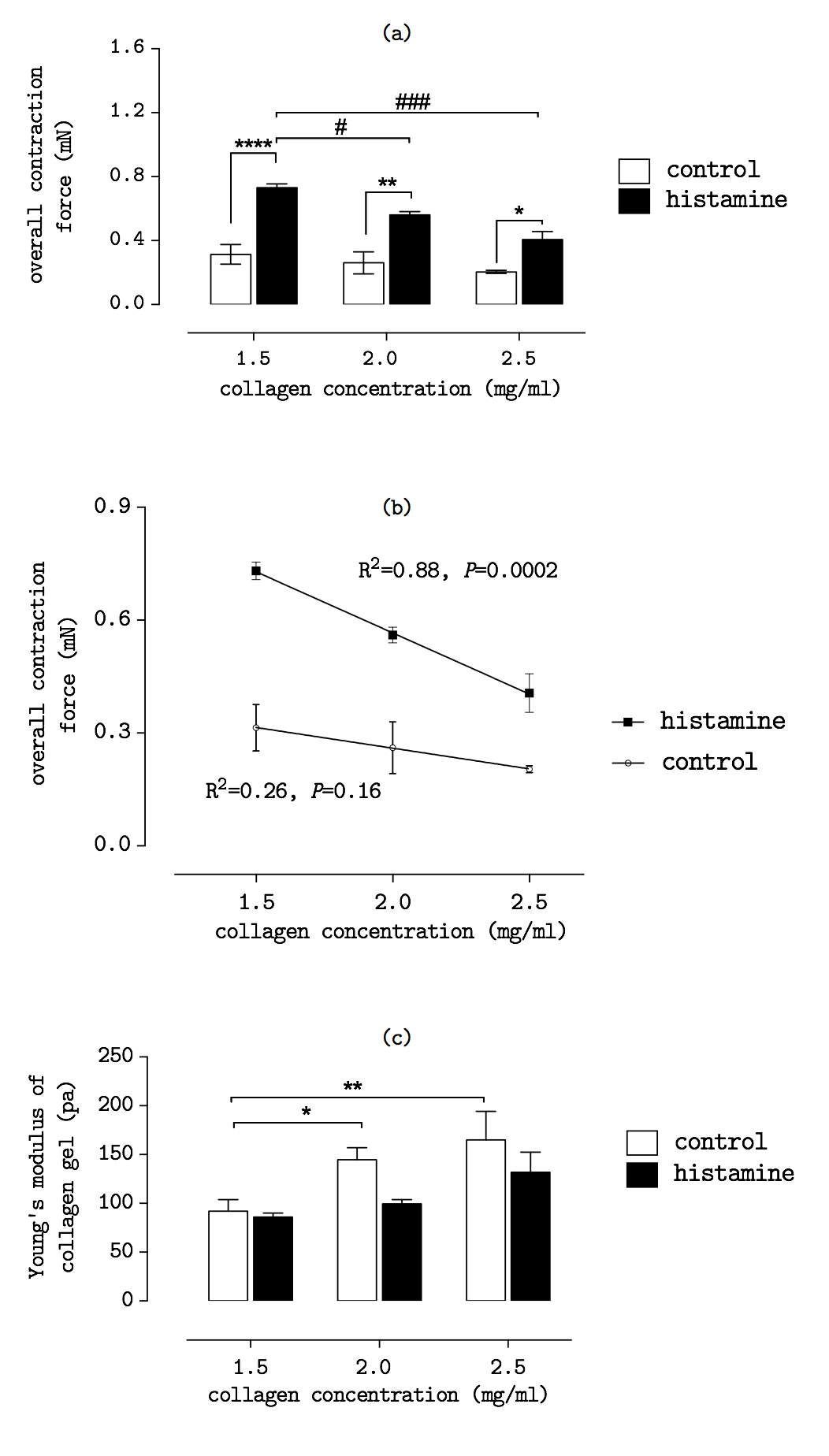
Conclusion: In 3D collagen matrix, the cells exhibit different contractility in response to the matrix stiffness change. The finding confirms the mechanical property of collagen matrix should be considered in the cellular contraction events induced by drugs. Overall the study has shown the importance of matrix stiffness in the design of collagen-based biomaterial for clinical applications.
References:
[1] Bell E, Ivarsson B, Merrill C. 1979 Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proceedings of the National Academy of Sciences 76, 1274- 1278.
[2] Montesano R, Orci L. 1988 Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proceedings of the National Academy of Sciences 85, 4894- 4897.
[3] Jin T, Li L, Siow RCM, Liu K-K. 2015 A novel collagen gel-based measurement technique for quantitation of cell contraction force. Journal of The Royal Society Interface 12, Article number 20141365