Introduction: Traumatic injury is the leading cause of death for both men and women between the ages of 5 and 44 worldwide, and blood loss is the primary cause of death at acute time points. Immediate intervention is critical to minimize mortality associated with severe trauma, and yet the only available treatments in the field are pressure dressings and absorbent materials which are effective for exposed wounds, but cannot address internal injuries. An intervention that could be administered intravenously and be stable at room temperature could fundamentally alter trauma care, save lives, and improve outcomes.
We have developed functionalized nanoparticles based on poly(lactic-co-gycolic acid) (PLGA), poly(ethylene glycol) (PEG), and the arginine-glycine-aspartic acid (RGD) peptide which binds the glycoprotein IIb/IIIa receptor. The receptor is only exposed on activated platelets. Our particles leverage the specificity of biology and are designed to help form stable clots faster to halt bleeding after intravenous administration.
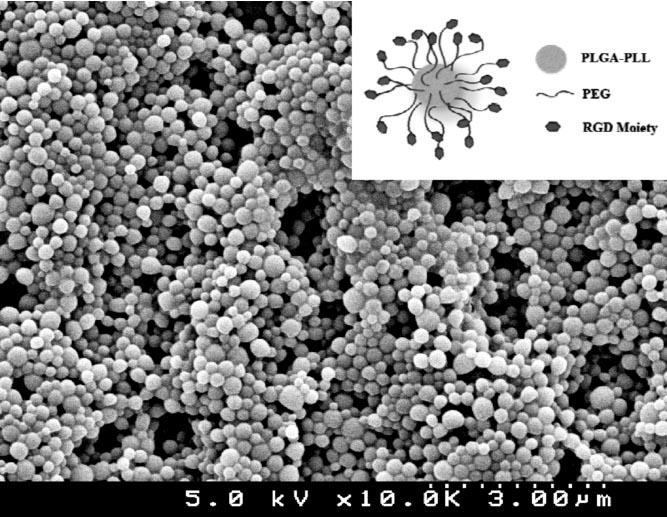
Experimental: Nanospheres were made using a precipitation process and their constituents were characterized using 1H-NMR, UV spectroscopy, amino acid analysis (AAA), scanning electron microscopy (SEM) and dynamic light scattering (DLS).
The efficacy of the hemostatic nanoparticles was tested in a femoral artery partial severance model as well as a a blunt liver injury model. Immediately following induction of injury, the surgical cavity is closed and hemostatic nanoparticles are administered. Control groups include no injection, saline injection, scrambled peptide sequence (inactive targeting sequence), and recombinant factor VIIa. An injection of 20 mg/ml hemostatic nanoparticles in PBS was used in the control group. Bleed times and bleed volumes are assessed using pre-weighted sponges to soak up blood. All major organs were harvested for histology and biodistribution of hemostatic nanoparticles. These models were augmented by a blast injury model of polytrauma and a model of brain injury with hemorrhaging.
Results and Discussion: The functionalized nanoparticle characterization was showed that particles were 154 +/- 36 nm by SEM and 345 +/- 81 nm hydrodynamic diameter by DLS. Chemical composition was confirmed using UV-vis and 1H-NMR and successful conjugation of the targeting ligand was verified using AAA.
The hemostatic nanoparticles halved bleeding time in the femoral artery model. In the liver injury model, we saw a reduction in blood loss and increased survival with the administration of the naoparticles. In both the blast injury and brain injury models, we see that the administration of the hemostatic nanoparticles reduces bleeding and this, in term leads to better behavioral outcomes. This suggests that reducing bleeding may be a critical component of providing neuroprotection after injury.
Conclusion: The functionalized nanoparticles or hemostatic nanoparticles reduce bleeding in a number of models of trauma. Following administration, the particles participate in clot formation and can be found at the injury site post injury. The chemistry of the formulation including the length of the PEG arms and peptide play significant roles in the efficacy and clearance of the particles and provide means to tailor the particle behavior. This treatment has the potential to greatly impact survival outcomes related to internal hemorrhage and may lead to better functional outcomes more broadly.
NIH Director’s New Innovator Award DP20D007338