Introduction: Liver tissue engineering aims to provide transplantable tissues to replace or regenerate liver tissue as a supplement to the nearly 16,000 patients currently awaiting transplants[1]. Hepatocytes are notoriously difficult to culture in vitro and require novel 3D scaffolds that can optimally support growth and function. Hepatocyte aggregation is known to have a positive influence on viability, however the influence scaffold pore size and shape has on aggregation and gene expression has yet to be fully investigated[2],[3]. Threee dimensional (3D) printing has been implemented in tissue engineering, and is particularly amenable to tissues of a repetitive nature such as the liver[4]. 3D printing allows for precise control over scaffold size, shape, porosity, and uniformity in a manner unrivaled in scaffold fabrication. Here we utilize 3D printed gelatin to demonstrate the effect varying scaffold pore size, shape, and tortuosity has on hepatocyte proliferation, viability, and gene expression.
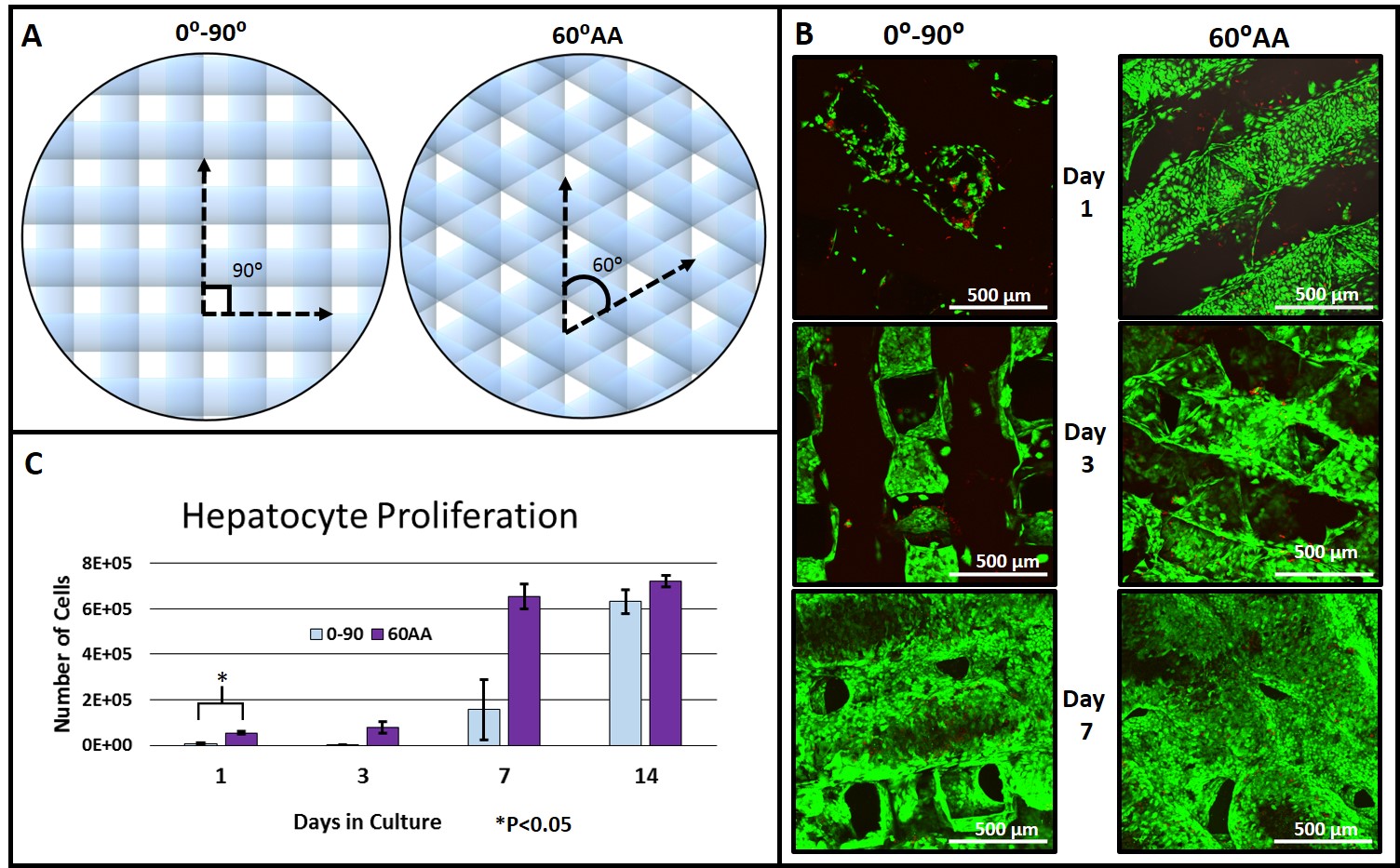
Materials and Methods: Porcine gelatin type A (10% w/v, PBS) was 3D printed using a 3D BioPlotter (EnvisionTEC GMBH, Germany) by heating the solution and extruding through a 200 μm diameter nozzle. Scaffold struts were spaced 650 μm apart, into 6 layer scaffolds with strut orientations of either 0⁰ and 90⁰ (0-90), or 0⁰, 60⁰, and 120⁰ (60⁰ advancing angle, 60AA). Figure 1 A shows a schematic. Scaffolds were then cross-linked with EDC/NHS and 4 mm diameter scaffold biopsy punches were seeded with HUH7 human hepatocytes and cultured in DMEM with 10% FBS. Six well culture plates were coated in gelatin and cultured as a control. Viability was imaged using live/dead (calcein/ethidium) and confocal microscopy. Proliferation was quantified using PicoGreen. Gene expression was assayed using qRT PCR. Relative expression levels were normalized to human cyclophilin 1.
Results and Discussion: The torturous pore geometry provided by 60AA scaffold architectures led to enhanced seeding efficiency, evident by cell numbers at day 1 (Figure 1 C) and imaging (Figure 1 B). Cell numbers were not significantly different between 60AA and 0-90 scaffolds on subsequent time points. However, viability imaging indicates significant differences in cell aggregation (Figure 1 B). By day 14, dense cell aggregates prevented useful image acquisition.
Gene expression data indicates little significant change from 2D culture at days 1 and 3, aside from albumin expression (Figure 2 A, B). By day 7, drastic changes in gene expression are observed, chiefly between the scaffolds of differing geometry (Figure 2 C). The primary differences are in expression levels of drug metabolism genes CYP3A4, CYP3A7, and CYP2C9. Drug metabolism is localized to pericentral hepatocytes, and tight pore geometries of 60AA scaffolds are hypothesized to mimic this environment by influencing cell packing[5]. These differences are suppressed after scaffold maturation in long term culture (Figure 2 D). Proliferation on the scaffold surface is hypothesized to dampen gene detection of those hepatocytes subject to the effects of differing pore geometry.

Conclusion: Despite no significant difference in cell number, gene expression levels of hepatocytes vary substantially depending or scaffold pore shape. These studies provide useful insight into optimal 3D printing schemes that may incorporate primary hepatocytes and co-cultures of non-parenchymal cells.
Alex Rutz
References:
[1] Organ Procurement and Transplantation Network (http://optn.transplant.hrsa.gov/)
[2] S. N. Bhatia, G. H. Underhill, K. S. Zaret, and I. J. Fox, “Cell and tissue engineering for liver disease,” vol. 6, no. 245, 2014.
[3] T. T. Chang and M. Hughes-Fulford, “Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes.,” Tissue Eng. Part A, vol. 15, no. 3, pp. 559–567, 2009.
[4] S. V Murphy and A. Atala, “3D bioprinting of tissues and organs,” Nat. Biotechnol., vol. 32, no. 8, pp. 773–785, Aug. 2014.
[5] P. Godoy, et al. "Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME." vol. 87, no. 8. 2013.