Introduction: Phenol-modified hydrogels for cell encapsulation are a growing area of research, although an appropriate initiator needs to be selected. A variety of chemical and enzymatic initiators have been used, with a range of results. Of note, radicals produced by chemical initiators have been shown to be cytotoxic[1]. Therefore, this work explores the use of enzymes to catalyse the crosslinking process. Many previous studies using enzyme crosslinkers use biological-based hydrogels. However many biological polymers can act as antioxidants, obscuring the effects of the initiating system on cells[1]. Therefore we chose poly(vinyl alcohol) (PVA) modified with tyramine (PVA-Tyr) groups to act as a “blank slate” material that does not interfere with the initiating system. The aim was to highlight both the advantages and challenges of four enzyme initiators. Specifically, the commonly used HRP/H2O2 was evaluated along with hematin/H2O2, laccase, and tyrosinase. These enzymes were evaluated for gel formation and cell response to encapsulation.
Materials and Methods: PVA-TYR was synthesised as described previously[2]. The initiators were added to the PVA-TYR solution (5% w/w) under gentle mixing. Initiators used were HRP (0-0.5 U/mL) with H2O2 (0-12 mM), hematin (0-0.08% w/w) with H2O2 (0-12 mM), laccase (0-25 U/mL) and tyrosinase (0-2000 U/mL). The samples were gelled at 37ºC and 5% CO2 for 4 hours.
The gelation kinetics were measured on a spectrophotometer for up to 6 hrs at 37°C. The wet and dry mass of the hydrogels was measured at 0 and 48 hrs to obtain the mass loss and swelling (q) of the hydrogels.
L929 murine dermal fibroblasts were added to the macromer solution prior to crosslinking at 1x106 cells/ml. Cell viability within gels was determined over one week of culture via a live/dead assay and the MTS assay.
Results: All of the enzymes evaluated can lead to the formation of highly crosslinked hydrogel networks. UV-Vis confirmed complete gelation for all enzymes within 4 hrs. A range of concentrations was tested for each enzyme to determine the concentration which produced the optimal gel, i.e., the lowest sol fraction. In all cases, the sol fraction was less than 30%, except for hematin which had 50% sol fraction, even at the highest concentrations. The optimal concentrations of enzymes were then used for the cell encapsulation studies. It is recognised that this method resulted in different concentrations of enzymes per system, but the goal was to keep the hydrogel network approximately the same, i.e., similar swelling and mesh size. The live/dead assay demonstrated that cells were alive in all systems initially; however there were stark contrasts in the viability and proliferation after 7 days, with tyrosinase and laccase outperforming the other two. Cell number was determined over 7 days by the MTS assay (See Figure 1), and further proved this finding.
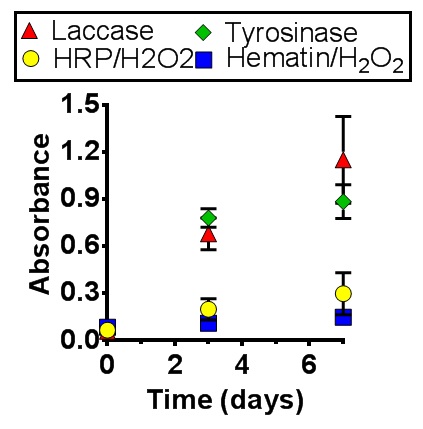
Conclusions: These results demonstrate that a thorough evaluation of enzyme initiators should be performed, as the initiator will impact the quality of the hydrogel formed and cellular proliferation, which is critical for biomedical applications.
The Whitaker foundation for a grant to JJ Roberts.
References:
[1] Lim, K.S., et al., Promoting Cell Survival and Proliferation in Degradable Poly(vinyl alcohol)-Tyramine Hydrogels. Macromol Biosci, 2015.
[2] Lim, K.S., et al., Covalent incorporation of non-chemically modified gelatin into degradable PVA-tyramine hydrogels. Biomaterials, 2013. 34(29): p. 7097-105.