Introduction: A precipitation reaction in ethylene glycol was recently shown to produce sub-micrometric, non-agglomerated β-tricalcium phosphate (TCP, Ca3(PO4)2) platelets of controllable aspect ratios with potential applications as fillers in load-bearing composites or as additives for enhanced injectability of pastes and cements[1]. X-ray diffraction (XRD) analysis showed a mismatch between the platelet crystal lattice and theoretical β-TCP. Since the crystal structure determines material properties including bioresorbability, the particularity of the platelet structure was scrutinized.
Materials and Methods: β-TCP platelets were precipitated from ethylene glycol solutions containing CaCl2 and H3PO4 (or Na2HPO4), which were pH-adjusted by NaOH or HCl and kept at constant temperature (90 to 170°C) for at least 30 min. The materials were analysed by Rietveld refinement of XRD patterns as well as by transmission Fourier transform infrared (FTIR) spectroscopy and inductively coupled plasma-mass spectroscopy (ICP-MS).
Results and Discussion: The electron density difference between the platelet crystal structure measured by XRD and the published (stoichiometric) β-TCP model[2] indicated reduced occupancies of the Ca(4) and O(2) crystallographic sites and splitting of the P(1) site (Fig 1A).
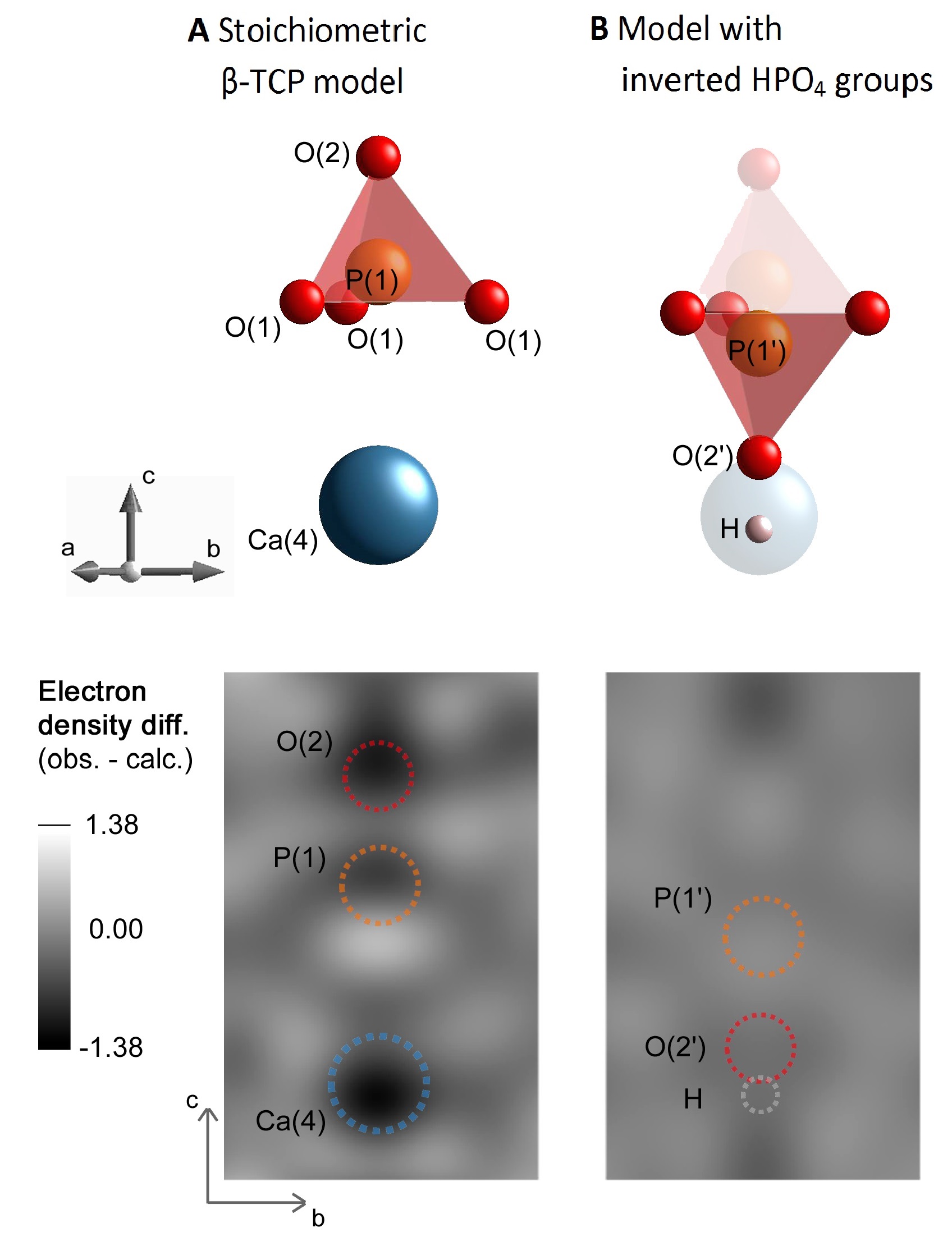
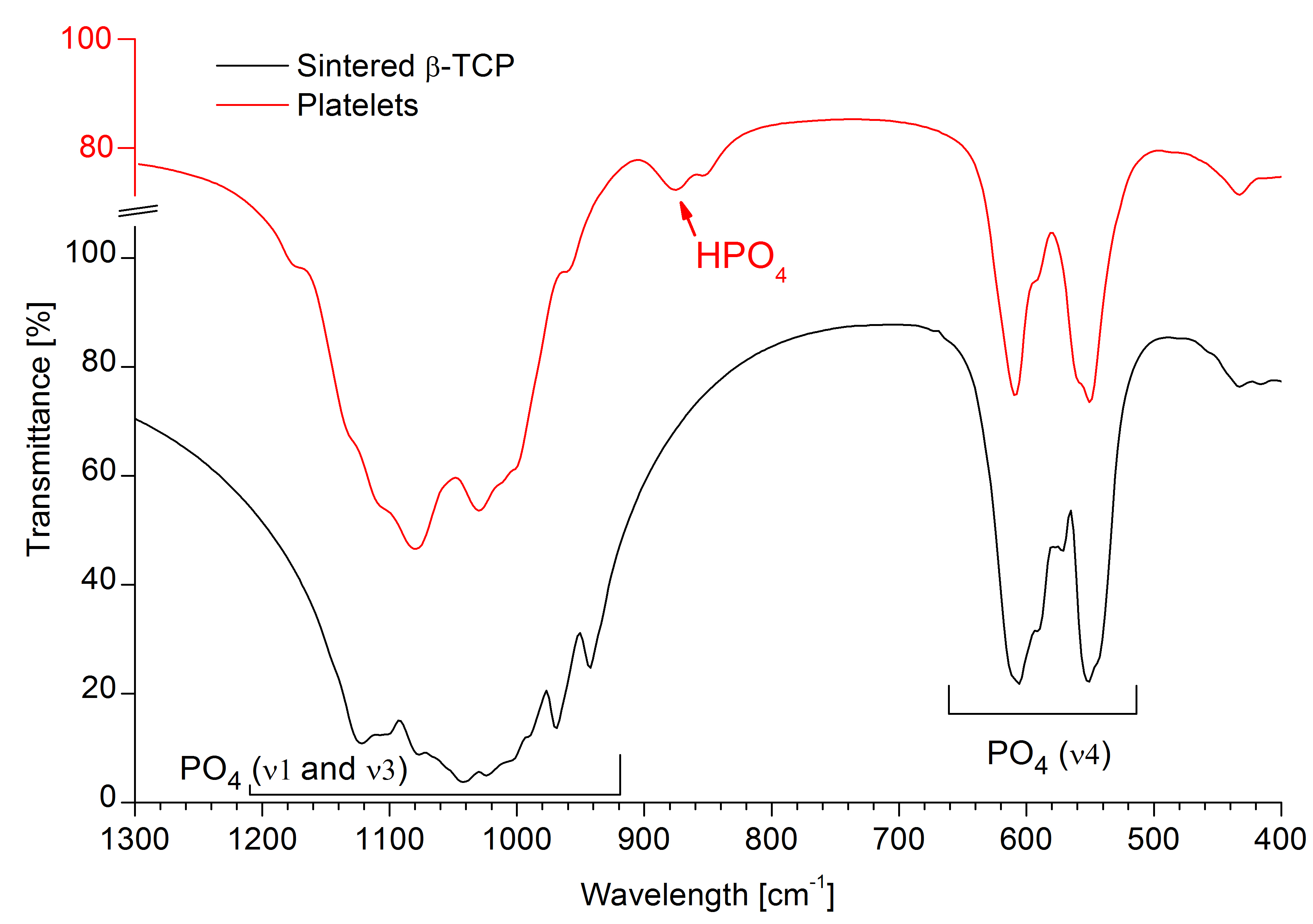
A similar arrangement has been observed in whitlockite, a Mg-containing β-TCP-like natural mineral, where the incorporation of HPO4 groups was proposed to compensate for cation deficiency[3]. Specifically, for each missing Ca(4) atom, two P(1)O4 tetrahedra are protonated and inverted about the plane of O(1) atoms, resulting in an electroneutral structure with mirrored P(1’), O(2’) and H positions (Fig. 1B, top). Adopting this model allowed for the refinement of the platelet structure with much smaller electron density mismatch compared to the published β-TCP model (Fig. 1B, bottom). An absorption band at 875 cm-1 in FTIR spectra confirmed the presence of HPO4 groups in platelets but not in sintered β-TCP (Fig. 2).
The Ca-deficit was corroborated by calcination of the platelets at 1000°C which led to decomposition into stoichiometric, H-free β-TCP (Ca/P = 1.5) and β-Ca2P2O7 (Ca/P = 1.0) where the global Ca/P ratio agreed closely with the initial β-TCP Ca/P ratio (ΔCa/P = 0.010) and with the elemental composition quantified by ICP-MS (ΔCa/P = 0.008). Specifically, the refinement determined a β-TCP Ca/P ratio of 1.444±0.003 (n=38) with no significant effects of synthesis parameters including temperature, time, pH, precursor Ca/P ratio and concentration.
Conclusions: This work describes a previously unknown H-substituted β-TCP structure, in other words a Mg-free whitlockite. An investigation of the degradation kinetics of this material is planned, due to the potential clinical benefit of a bone substitute material exhibiting a higher solubility than conventional β-TCP.
Swiss National Science Foundation (SNF; 200021_13758)
References:
[1] L. Galea et al, Biomaterials 2013; 38:6388-6401
[2] B. Dickens et al, J Solid State Chem 1974; 10, 232-248
[3] C. Calvo et al, Am Mineral 1975; 60(1/2):120-133