Tendon reattachment using demineralised bone matrix and mesenchymal stem cells
-
1
UCL, Institute of Orthopaedics and Musculoskeletal Science, United Kingdom
-
2
Royal National Orthopaedic Hospital, United Kingdom
Introduction: Failure of tendon-bone healing is still problematic though, and occurs in up to 94% of cases. A number of novel tissue engineering strategies have been investigated with scaffolds and biologically engineered materials emerging as the most effective. Demineralised bone matrix (DBM) promotes healing of the tendon-bone interface[1] but its role in the treatment of tendon tears with retraction has not been investigated. The purpose of this study was to evaluate the effect of allogenic and xenogenic DBM incorporated with autologous mesenchymal stem cells (MSCs) on regeneration of the tendon-bone interface in an ovine model of tendon retraction. Since xenografts are cheaper and more readily available than their allogenic derivatives we wanted to establish their role in tendon-bone healing. We hypothesized that augmentation of a healing tendon-bone interface with DBM incorporated with autologous MSCs would result in improved function, and restoration of the native enthesis, with no difference between xenogenic and allogenic scaffolds.
Methods: Animal care and experimental procedures were conducted in accordance with a Project License protocol accepted under the UK Home Office Animals (Scientific Procedures) Act 1986. The patellar tendon was detached and a complete distal tendon defect measuring 1 cm was created. Freeze dried DBM derived from cortical strips of tibial bone was made according to the protocol of Urist[2]. Suture anchors were used to reattach the shortened tendon, and xenogenic DBM + MSCs (n=5) and allogenic DBM + MSCs (n=5) were used to bridge the defect. In all animals the buffy coat obtained from a bone marrow aspirate was used at the time of surgery and was incorporated within and onto the DBM scaffold. Functional recovery was assessed at 6, 9 and 12 weeks. DBM incorporation into the tendon and its effect on regeneration of the enthesis was measured using histomorphometry. Three distinct morphological zones within the regenerating enthesis were evaluated by two blinded observers. Zone 1: DBM-patellar tendon interface; Zone 2: DBM in the tendon defect; Zone 3: DBM neo-enthesis. Histology was assessed semi-qualitatively for new bone formation, inflammatory cells, cellularity, vascularization and collagen fibre crimp. Mann Whitney U tests were used to assess differences within each group over time. Results were considered significant at the p > 0.05 level.
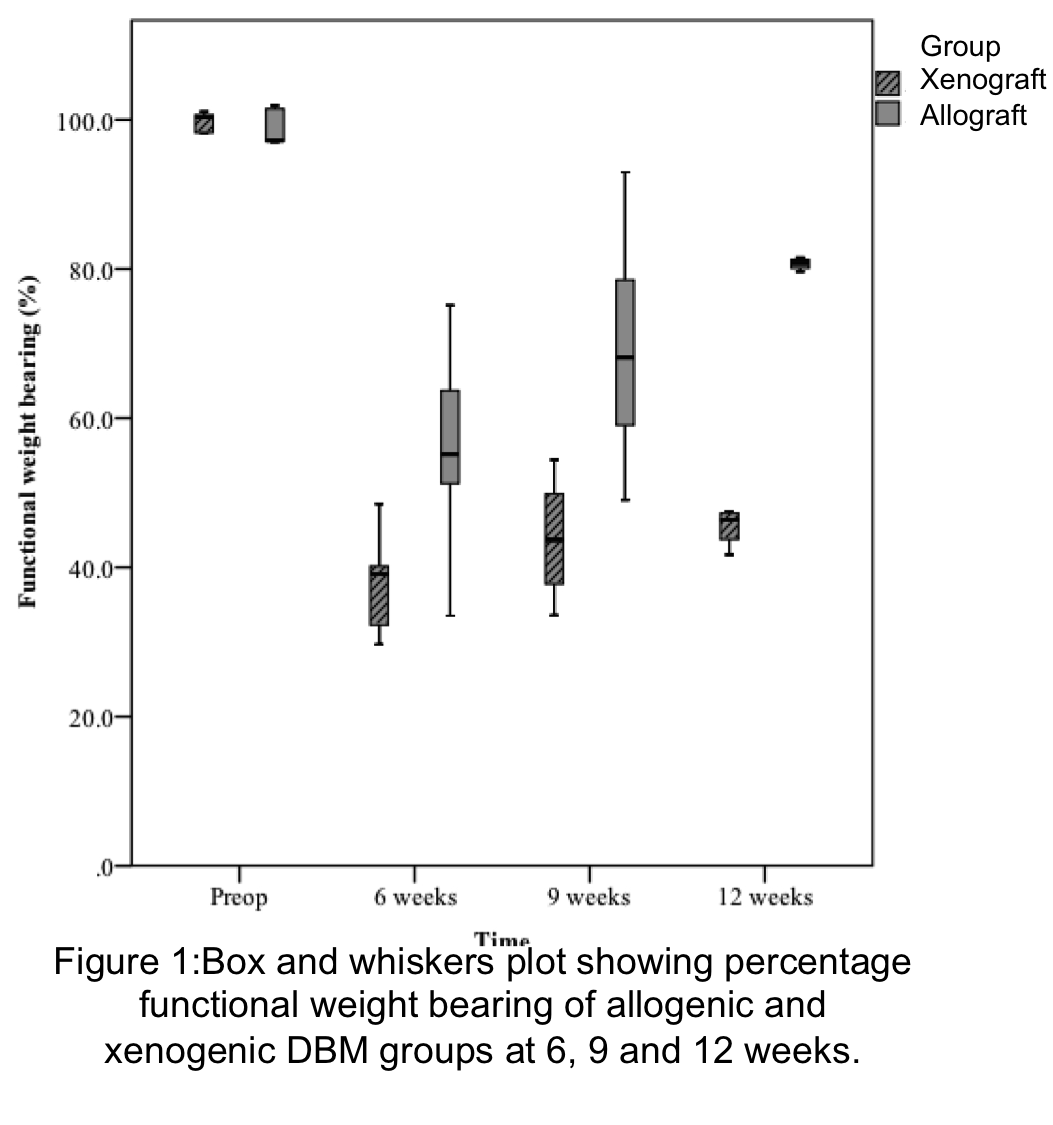
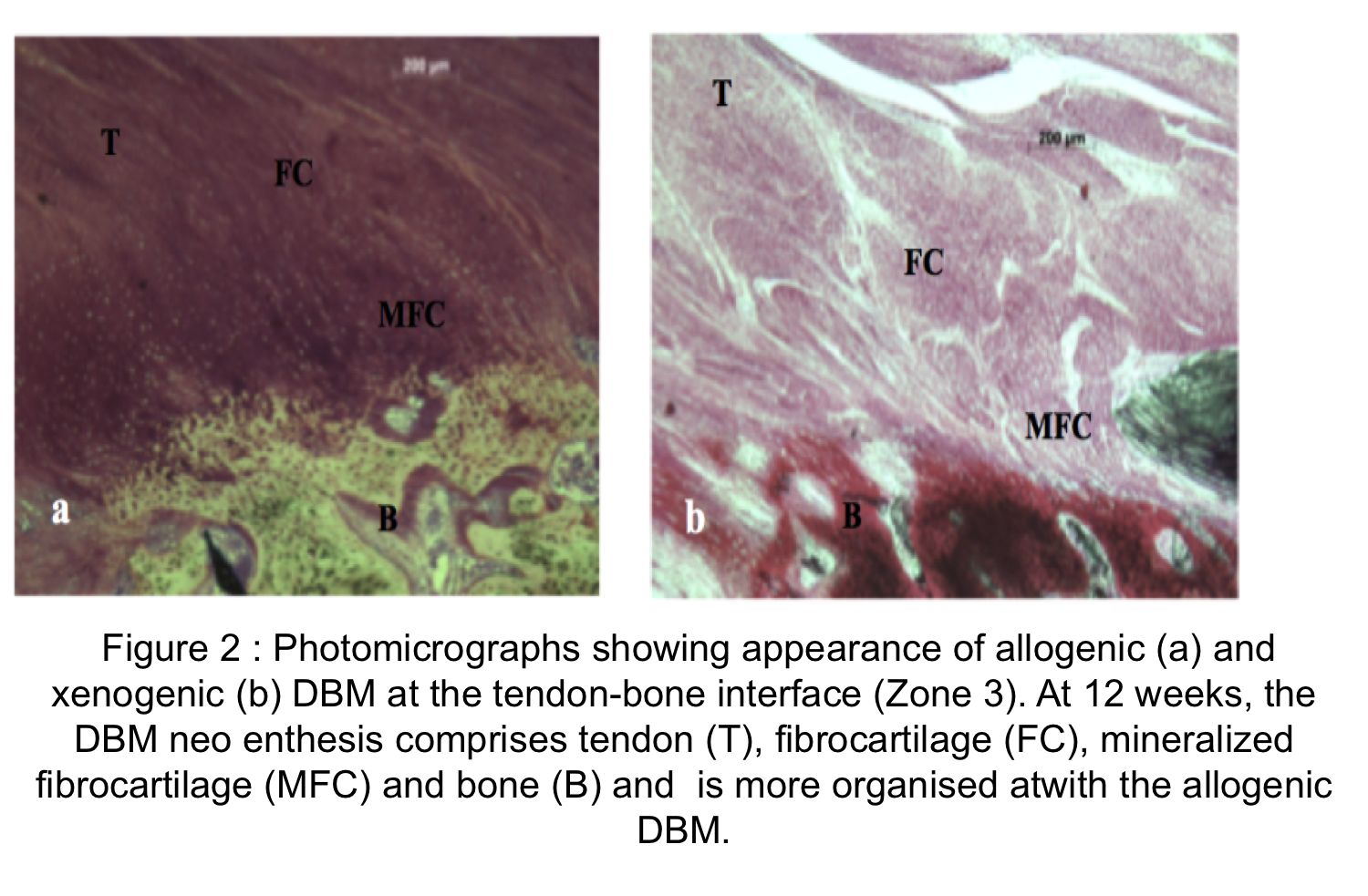
Results: Between 6, 9 and 12 weeks, DBM augmentation significantly improved functional weight bearing with no failures in either group. Compared to xenogenic DBM, the allograft group was associated with significantly higher functional weight bearing at 6 (P=0.047), 9 (P=0.028) and 12 weeks (P=0.009)(Figure1). Semi quantitative analysis showed that compared to xenogenic DBM, the allograft group was significantly more remodeled with tendon-like tissue in the region of the defect (p=0.015). In both groups the tendon-bone interface (Zone 3), was accompanied by mineralized fibrocartilage consisting of chondrocytes surrounded by a mineralized matrix. This direct type of enthesis, with a characteristic transition between bone, mineralized fibrocartilage, demineralized fibrocartilage and tendon was seen in both groups however, xenogenic DBM, showed fewer regions of mineralized fibrocartilage at the tendon-bone interface. A more direct type of enthesis characterized by significantly more fibrocartilage (p=0.033) was measured in the allograft group (Figure 2).
Discussion: In a model of tendon retraction allogenic and xenogenic DBM can regenerate the tendon-bone interface. This was more mature and organized in the allograft group giving better functional results. We speculate that DBM’s ability to produce cartilage and bone through endochondral ossification is responsible for its role in regeneration of the enthesis. The superior biomechanical and histological results noted in the allogenic group, highlight its potential to be used in the clinical setting to augment rotator cuff tendon-bone healing.
References:
[1] Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
[2] Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
Keywords:
Regenerative Medicine,
in vivo,
Scaffold,
in vivo tissue engineering
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Biomaterials to modulate biological processes involved in host response
Citation:
Shahbazi
S,
Thangarajah
T,
Pendegrass
C,
Lambert
S,
Alexander
S and
Blunn
G
(2016). Tendon reattachment using demineralised bone matrix and mesenchymal stem cells.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01485
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Shirin Shahbazi, UCL, Institute of Orthopaedics and Musculoskeletal Science, London, United Kingdom, shirin.shahbazi.13@ucl.ac.uk
Dr. Tanujan Thangarajah, UCL, Institute of Orthopaedics and Musculoskeletal Science, London, United Kingdom, tanujan.thangarajah.13@ucl.ac.uk
Dr. Catherine Pendegrass Pendegrass, UCL, Institute of Orthopaedics and Musculoskeletal Science, London, United Kingdom, c.pendegrass@ucl.ac.uk
Dr. Simon Lambert, UCL, Institute of Orthopaedics and Musculoskeletal Science, London, United Kingdom, simonlambert@me.com
Dr. Susan Alexander, UCL, Institute of Orthopaedics and Musculoskeletal Science, London, United Kingdom, drsalexander@me.com
Dr. Gordon Blunn, UCL, Institute of Orthopaedics and Musculoskeletal Science, London, United Kingdom, g.blunn@ucl.ac.uk