Introduction: The regeneration of infected segmental bone defects is a long-standing challenge in the orthopedic field. Local delivery of antibiotics is an attractive solution due to increased local drug concentrations and decreased systemic toxicity. However, many antibiotics are also specifically toxic to bone cells and present a barrier to bone regeneration[1]. In this study, we investigate the controlled release of clindamycin hydrochloride as a pro-osteogenic molecule. We hypothesize that clindamycin promotes bone healing through induction of osteogenic differentiation of human mesenchymal stem cells (hMSC) in vitro and that controlled release of this drug from poly(lactic-co-glycolic acid) (PLGA) microparticles in a 3 mm rat femoral defect will increase bone bridging and regenerated bone volume.
Materials: PLGA (Evonik Industries), clindamycin HCl (TCI America), polycaprolactone (PCL) (Sigma), and PCR reagents (Affymetrix, Promega) were obtained. The hMSCs cells isolated from healthy human bone marrow were provided by M.D. Anderson Cancer Center. Rats were purchased from Charles River Laboratories, and custom fixation devices were acquired from Georgia Institute of Technology.
Methods: Primary hMSCs were seeded onto 3 mm electrospun PCL scaffolds for DNA, alkaline phosphatase, and calcium quantitative assays (n=4/group) and onto 8 mm scaffolds for PCR analysis (n=3/group). Cells were treated with 500, 100, 50, or 0 ug/mL clindamycin HCl (CL500, CL100, CL50, and CL0, respectively) for 28 days. Lewis rats received a unilateral 3 mm subcritical size femoral defect treated with either unloaded (n=8) or clindamycin-loaded (n=7) PLGA microparticles[2]. The clindamycin group received a total dose of 10 mg; both groups received the same total mass of microparticles. After 12 weeks, femurs were harvested and evaluated by microcomputed tomography and histology.
Results and Discussion: In hMSCs cultured in vitro, CL500 demonstrated reduced cell proliferation compared to all other groups, but cells recovered the ability to proliferate after day 14 (Fig. 1A). Furthermore, quantitative calcium assay demonstrated that the CL500-treated group produced significantly more calcium than CL0, CL50, or CL100 groups (Fig. 1B-C).
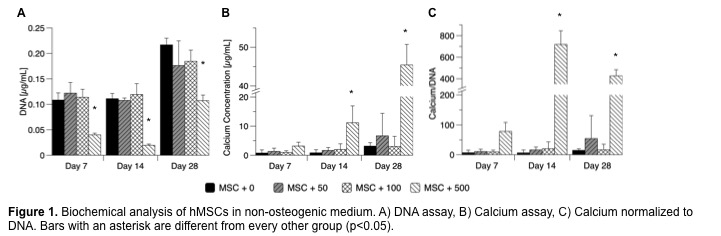
Preliminary PCR results indicated that the CL500-treated MSCs produced >100-fold increase in osteogenic transcription factors Osx and Dlx5 at day 14 as compared to other treated cells, suggesting that CL500 is inducing mineralization of hMSCs that may be driven by osteogenic differentiation. Rats treated with 10 mg of locally delivered clindamycin showed increased bony bridging across the segmental defect as well as increased regenerated bone volume compared to defects treated with blank PLGA microparticles (p<0.05) (Fig. 2).
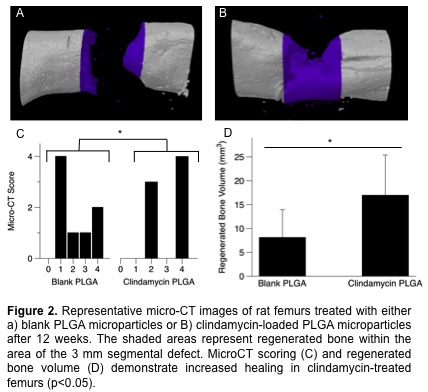
This pilot study demonstrates that the results seen in vitro are translatable to an in vivo model of bone regeneration.
Conclusions: Because infection is a major complication of bone injury, antibiotics play a major role in the local milieu surrounding regenerating bone. While most antibiotics show either no or deleterious effect on bone regeneration, we demonstrate here that local delivery of clindamycin via PLGA microparticles may be a judicious candidate for prevention and/or treatment of long bone osteomyelitis since clindamycin is a drug that could serve the dual functions of (i) killing bacteria and (ii) stimulating bone regeneration.
Natasja van Dijk; Patrick P. Spicer; Ruth L. Kirschstein Fellowship from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (F30 AR067606)
References:
[1] Rathbone CR, Cross JD, Brown KV, Murray CK, Wenke JC. Effect of Various Concentrations of Antibiotics on Osteogenic Cell Viability and Activity. J Orthop Res. 2011;29:1070–4.
[2] Shah SR, Henslee AM, Spicer PP, Yokota S, Petrichenko S, Allahabadi S, Bennett GN, Wong ME, Kasper FK, Mikos AG. Effects of antibiotic physicochemical properties on their release kinetics from biodegradable polymer microparticles. Pharmaceutical Research. 2014;31:3379–89.