Introduction: Blood circulation persistence is a key determinant of tumor biodistribution of IV-delivered siRNA nanomedicines. We recently reported that PEGylated siRNA nanopolyplexes (si-NPs) with balanced cationic and hydrophobic content in the core have increased stability and in vivo blood circulation half-life[1] and that siRNA hydrophobization by lipid conjugation increases loading and potency of polymer-based nanocarriers[2]. Here, we investigated the benefit of hydrophobic interactions between siRNA cargo and PEGylated polymeric carriers for IV RNAi cancer therapies.
Methods: Loading efficiency, cell uptake, and stability against destabilization by herapin sulfate were assessed for NPs loaded with unmodified siRNA or siRNA-PA (si-NPs and siPA-NPs, respectively). The in vivo circulation half-life, tumor accumulation, and silencing efficacy were evaluated in a mouse orthotopic breast tumor model. For knockdown studies, the mice were dosed IV at 0 and 24 hrs.
Results and Discussion: siPA-NPs exhibited superior complexation efficiency compared to si-NPs (~4-fold less polymer needed) as well as greater heparin stability (siPA-NPs were stable at ~2-fold higher heparin concentration). These data indicate that hydrophobization of siRNA stabilizes polyplexes by introducing hydrophobic interactions between the cargo and the core polymer components, thus protecting against dissociation by competing polyanions. Additionally, the in vivo circulation half-life was ~2-fold higher for siPA-NPs, corresponding to a >2-fold increase in tumor accumulation in a murine tumor model (Figure 1A). The siPA-NPs produced luciferase silencing in tumors after 1 treatment, whereas si-NPs required 2 treatments (at 0 and 24 hrs) to produce significant silencing (Figure 1B). These results strongly support the effectiveness of siPA-NPs for target gene silencing via IV administration.
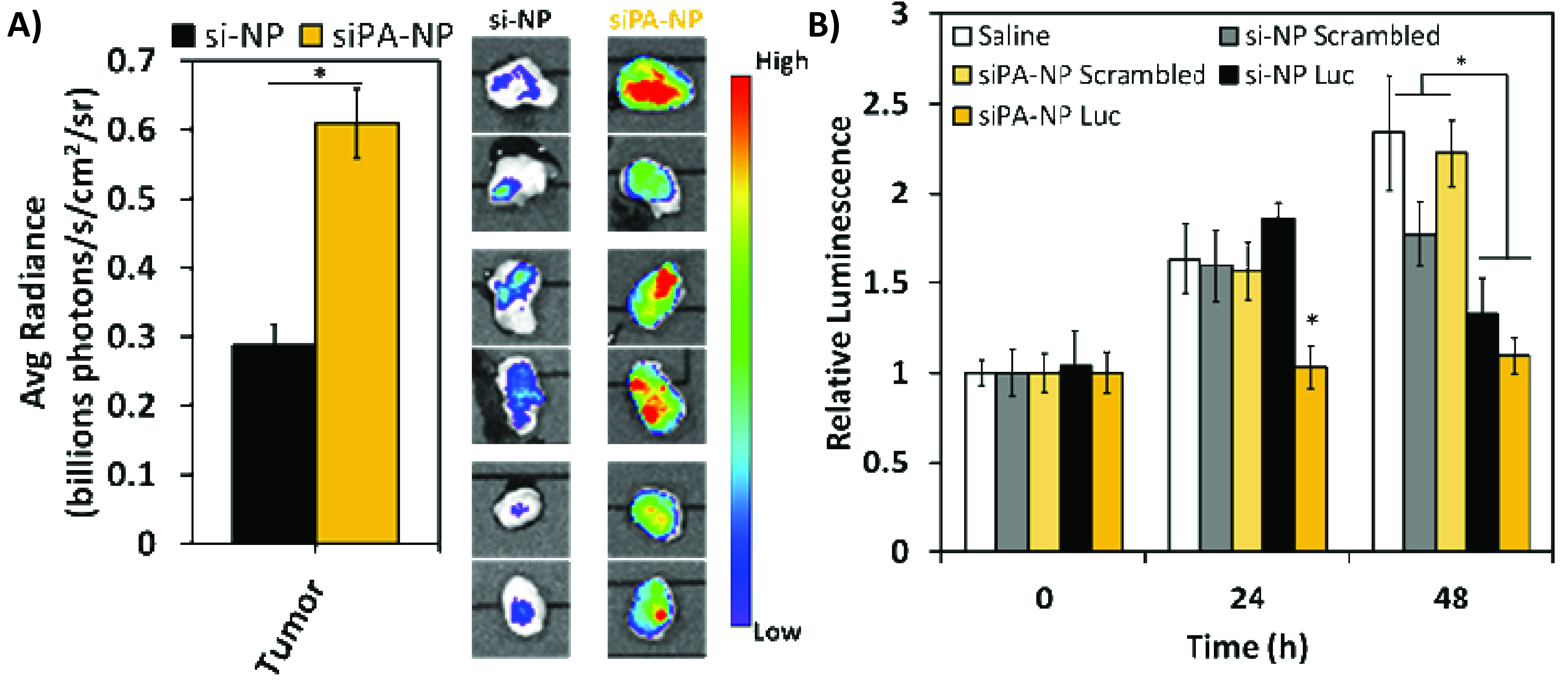
Figure 1. siPA-NPs improve accumulation (A) and luciferase silencing (B) at tumor site relative to si-NPs.
Conclusions: Our results demonstrate that engineering a mechanism for hydrophobic interactions between siRNA and its nanocarrier (in addition to the electrostatic forces that traditionally drive siRNA/polyplex loading) improves bioactivity in vivo. This is highly relevant for IV-delivered siRNA delivery platforms, as siRNA polyplexes often undergo rapid renal clearance due to destabilization by the heparin sulfates in the kidney. Our findings demonstrate that the underexplored approach of tuning the core interactions of polyplexes is an exciting strategy for increasing translatability of systemic RNAi cancer therapies.
References:
[1] Nelson CE, Kintzing RJ, Hanna A, Shannon JM, Gupta MK, Duvall CL. Balancing cationic and hydrophobic content of PEGylated siRNA polyplexes enhances endosome escape, stability, blood circulation time, and bioactivity in vivo. ACS Nano 7, 8870-8880 (2013).
[2] Sarett SM, Kilchrist KV, Miteva M, Duvall CL. Conjugation of palmitic acid improves potency and longevity of siRNA delivered via endosomolytic polymer nanoparticles. Journal of Biomedical Materials Research. Part A, doi:10.1002/jbm.a.35413 (2015).