Introduction: Complex structural organization in the human body is a key factor to produce the appropriate tissue functionality. Therefore, a technique for mimicking microstructures in native tissues is important to achieve engineering biomimetic functional tissues. Particularly in mature skeletal muscle, the muscle fibers are highly oriented and the anisotropic structure is essential to produce its mechanical functions. Thermoresponsive poly(N-isopropylacrylamide) (PIPAAm)-grafted surfaces allow the production of a tissue-like cell monolayer, a "cell sheet", and our group have developed this tissue engineering strategy for application in regenerative medicine. In this study, using a micropatterned thermoresponsive substrate, we have engineered 3D muscle tissues with well-organized microstructures.
Materials and Methods: The original procedures for the preparation of micropatterned thermoresponsive surfaces have been reported previously[1]. Briefly, thermoresponsive PIPAAm was grafted on a glass substrate, and then hydrophilic poly(N-acryloylmorpholine) was further polymerized spatio-selectively through a conventional photolithographic process. Human skeletal muscle myoblasts were seeded onto the patterned substrate (stripe patterns of two different polymer regions: 50 um / 50 um). The myoblasts were cultured until reaching confluence at normal culture temperature (37°C) and then temperature was lowered to 20°C for harvesting cells as a single continuous cell sheet. To produce a 3D tissue construct, multiple cell sheets were layered using a gelatin gel-coated manipulator. Furthermore, within this multilayered cell sheet construct, human induced pluripotent stem (iPS) cell-derived neurons and human umbilical vein endothelial cells (HUVECs) were incorporated for mimicking native muscle tissues. Specifically, these cells were sandwiched between multiple myoblast sheets. In addition, to investigate the contractile activity, the engineered tissue was cultured for 3 weeks in differentiation medium and then stimulated electrically.
Results and Discussion: Myoblasts were aligned on the surface and harvested as an anisotropic cell sheet by lowering temperature (Fig. 1a, b). By layering these cell sheets, a 3D oriented myotube construct was produced without 3D scaffolds (Fig. 1c)[2]. After sandwiching neurons and endothelial cells between layered cell sheets, both type of cells were aligned with the myoblast orientation and finally formed oriented networks in the construct (Fig. 2). Importantly, the presence of the top cell sheet triggered cellular signaling in the endothelial cells and allowed the formation of a vascular-like branching structure. This indicated that the cells recognized the anisotropy of the 3D environment and self-organized the microstructures in the construct. On the other hand, by electrical stimulation, contraction of the construct was observed. The contractile activities were synchronized with the electric pulse.
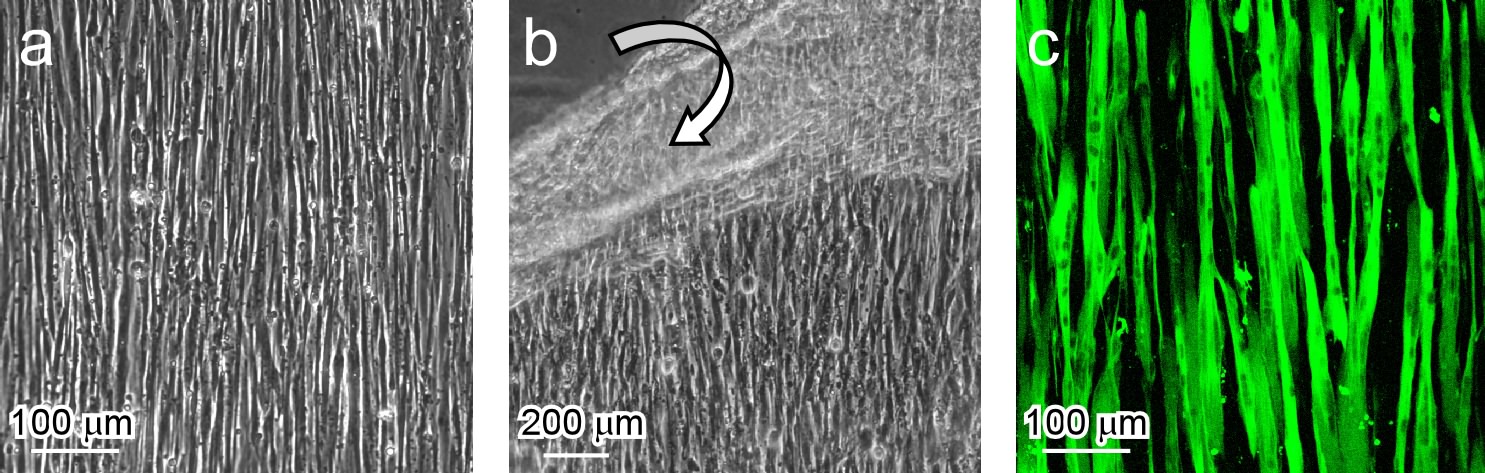
Figure 1. (a) Myoblasts aligned on the micropatterned thermoresponsive surface. (b) Detachment of an anisotropic cell sheet by lowering culture temperature. (c) An aligned myotube construct produced by culturing an anisotropic myoblast sheet in differentiation medium (2% horse serum).
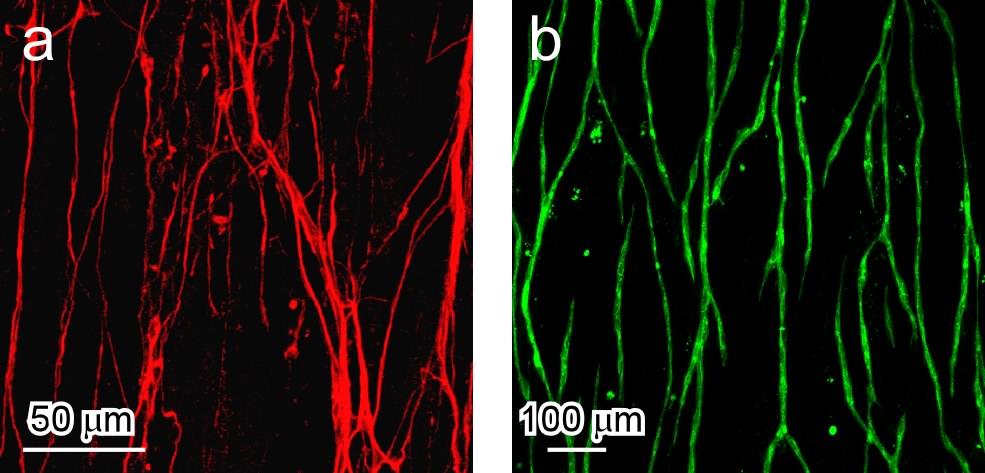
Figure 2. Formation of anisotropic cellular networks in multilayered myoblast sheets. Neurons (a) or endothelial cells (b) were sandwiched between two anisotropic myoblast sheets. At Day 5, confocal microscopic images were taken.
Conclusion: We have developed a novel technique to create a muscle tissue construct based on anisotropic tissue units. Uniquely in this scaffold-free tissue construct, neurons and endothelial cells self-organized their cellular networks. In addition, aligned myotubes showed contractile activities. The structural design and functionalization could lead to truly biomimetic tissue generation, and development of in-vitro physiological tissue models.
References:
[1] Takahashi H. et al. Biomacromolecules. 2011; 12: 1414-1418.
[2] Takahashi H. et al. Biomaterials. 2013; 34: 7372-7380.