Corrosion behavior of new beta type Ti-29Nb-13Ta-4.6Zr alloy in simulated body fluid solution
-
1
Andalas University, Kampus Unand Limau Manis, Mechanical Engineering Dept, Indonesia
-
2
Graduate School of Engineering, Osaka University, Division of Materials and Manufacturing Science, Japan
-
3
Tohoku University, Institute for Materials Research, Japan
-
4
Laval University, Dept. Mining, Metallurgical and Materials Engineering & CHU de Québec Research Center, Canada
Introduction: A new β type Ti alloy, Ti-29Nb-13Ta-4.6Zr (TNTZ), with low Young's modulus (~58 GPa) has been recently developed for anti-bone absorption implant having low stress shielding impact [1]. Its mechanical properties can be further modulated by performing heat or thermo-mechanical treatments [1],[2]. This study aims to preliminarily assess the yet unknown corrosion behavior of this novel alloy in simulated body fluid as one of the important parameters in view of its application for medical implants.
Materials and Methods: TNTZ alloy (31.5Nb, 11.6Ta, 4.7Zr, 0.03Fe, <0.02Al and bal, all in wt.%) was prepared by levitation melting. Samples of the alloy and Ti6Al4V (control) were polished (up to grit #1200) and subjected to corrosion test (surface area 10 x 10 mm2) in simulated body fluid as suggested by Kokubo [3] at pH 7.4 and 37oC. Corrosion tests were conducted by using a Versastat-3 potentiostat with three electrodes system where Ag/AgCl, graphite and the specimens served as the reference, counter and working electrodes, respectively. The specimens were stabilized at open circuit potential (OCP) for 30 min and continued with polarization at a scan rate of 2 mV/s from -1 V to 1.5 V. The corrosion potential (Ecorr), corrosion current (icorr), and corrosion rate were determined by Tafel fit according to the ASTM G102 standard. The surface and cross section of the specimens after the tests was examined by using optical microscope and SEM, EDX and XPS.
Results and Discussion: Fig. 1 shows typical potentiodynamic polarization curves for TNTZ specimens. Based on Tafel fitting, the average Ecorr, icorr and corrosion rate of TNTZ and Ti6Al4V, respectively are: -0.4 V, 0.2 V; 2x10-7 A/cm2, 3x10-8 A/cm2; and 1.2x10-9 mm/year, 3x10-10 mm/year. It indicates a slight decrease of corrosion resistance of TNTZ compared to Ti6Al4V alloy. However, its corrosion rate is still far lower than that of 316L type stainless steel (2.1x10-3 mm/year in Ringer’s solution and 1.5x10-4 mm/year in Hanks’ solution) [4], and that of Ti-Ni alloy (4.6 x 10-4 mm/year in Hanks’ solution) [5].
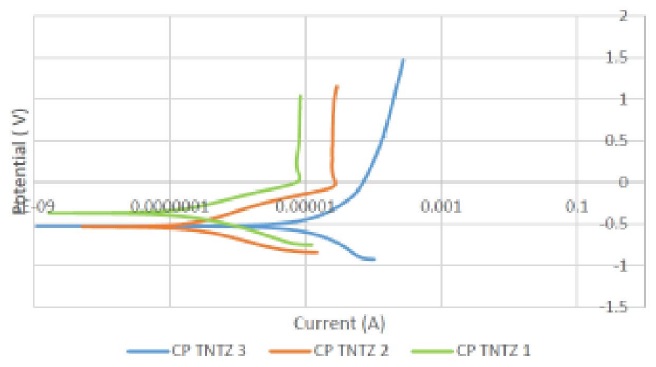
Fig. 1: Typical potentiodynamic polarization curves for TNTZ specimens.
Fig. 2 shows evidence of pitting corrosion in TNTZ specimen after subjected to the corrosion test. Further optical microscope examination on the cross sectional area confirm the pitting in the forms of elliptical and undercutting. Chemical compositional examination by EDX near the pitting area revealed higher oxygen content in both alloys with wider distribution on TNTZ without the trace of Zr. It is suspected that Zr was released/leached into the solution provoking pitting. Further analysis by XPS revealed higher binding energy and intensity for O and Ti than for Nb, Zr and Ta indicating the formation of predominantly TiO2 layer on the surface of TNTZ. We suspect micro segregation as the cause for pitting corrosion as the alloy was not homogenized adequately after casting.
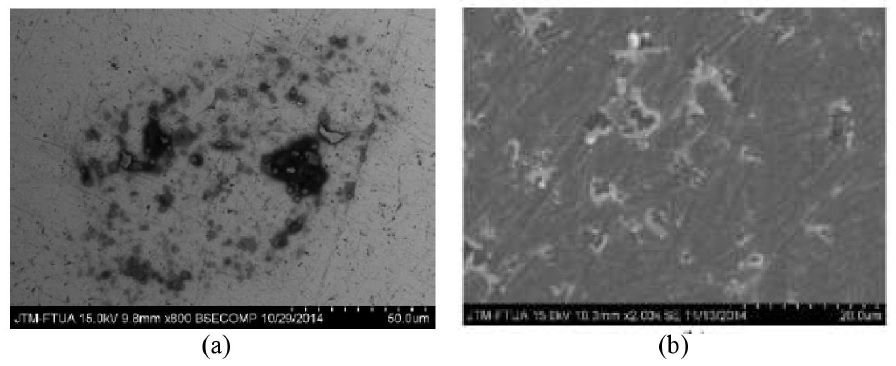
Fig. 2: Surface morphology of: (a) TNTZ, and (b) Ti6Al4V specimens after corrosion test.
Conclusion: This preliminary corrosion study reveals the high corrosion resistance of TNTZ alloy but with a tendency of pitting corrosion. The alloy should be subjected to further treatment such as homogenization to improve its corrosion behavior.
The authors acknowledge Andalas University for student travel grant, Universiti Teknologi Malaysia for corrosion test facility and Institute for Materials Research for TNTZ samples.
References:
[1] M . Niinomi, Metallurgical and Materials Transactions A 2002;33:477–486.
[2] N. Sakaguchi, et-al., Materials Science and Engineering C 2005;25:370-376.
[3] T. Kokubo, H. Takadama,Biomaterials. 2006;27:2907-2915.
[4] M. Talha, et-al.,, Journal of Chemical Pharmaceutical Research 2012;4:203-208.
[5] J. Ryhanen, et-al., Journal of Biomedical Materials Research 1998;41:481-488.
Keywords:
biomaterial,
corrosion,
material design,
Novel material
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Metallic biomaterials and alloys
Citation:
Gunawarman
G,
Refieska
A,
Ilhamdi
I,
Affi
J,
Cho
K,
Nakai
M,
Hermawan
H and
Niinomi
M
(2016). Corrosion behavior of new beta type Ti-29Nb-13Ta-4.6Zr alloy in simulated body fluid solution.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01060
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Andrew Refieska, Andalas University, Kampus Unand Limau Manis, Mechanical Engineering Dept, Padang, Indonesia, Email1
Dr. Ilhamdi Ilhamdi, Andalas University, Kampus Unand Limau Manis, Mechanical Engineering Dept, Padang, Indonesia, Email2
Dr. Jon Affi, Andalas University, Kampus Unand Limau Manis, Mechanical Engineering Dept, Padang, Indonesia, Email3
Dr. Maasaki Nakai, Tohoku University, Institute for Materials Research, Sendai, Japan, Email4
Dr. Hendra Hermawan, Laval University, Dept. Mining, Metallurgical and Materials Engineering & CHU de Québec Research Center, Québec City, QC, Canada, Email5
Dr. Mitsuo Niinomi, Tohoku University, Institute for Materials Research, Sendai, Japan, Email6