Background: Wear of metal-on-metal (MoM) bearings results in the release of metal debris into the surrounding tissues leading to adverse reactions to metal debris (ARMD). In vitro hip simulators failed to predict high rates or wear and cannot predict ARMD identified with certain designs of MoM bearings in clinical studies. For this reason, we have developed an ovine total hip arthroplasty model where the metal ion release and biological response can be measured. Our hypothesis was that in this model a 4th generation ceramic SLC coating applied to the femoral head and acetabular cup surfaces would result in a reduction in volumetric wear, a decrease in blood chromium and cobalt ion levels and reduce any potential ALTRs when compared to MoM combinations.
Methods: 3 groups of implants were used: 1) MoM with a SLC ceramic coating (SLC-MoM), 2) uncoated MoM surfaces (MoM) and 3) a metal on polyethylene (MoP) bearing. Implants were inserted into male sheep. Implants remained in vivo for 13 months during which time the sheep were regularly exercised to make sure the implants received a minimum level of use. Bearing surface and taper measurements to assess surface roughness (Ra and Rz) and volumetric wear were obtained pre- and post operatively using a Co-ordinate Measuring Machine (CMM), a Taylor Hobson Roundness machine, an Optical 3D Surface Profilometry Microscope and a Scanning Electron Microscope (SEM). Blood chromium (Cr) and cobalt (Co) metal ion levels were measured pre-operatively and at 2 and 4 weeks, and 4, 6, 12 and 13 months post surgery. MRI was used to investigate joint effusion, capsule thickness and periprosthetic masses. Samples of synovial tissue, the testes, spleen, heart, brain, kidney, liver, lung and bladder wall were obtained. Tissues were processed for histological analysis and graded using an ALVAL scoring system.
Results: SEM showed the SLC coating to be durable with no signs of delamination, coating loss or adverse reactions. No significant differences in Ra and Rz values were seen when taper and bearing surface roughness were compared. Significantly lower levels of Cr were measured in both the SLC-MoM group (p = 0.010) and MoP group (p = 0.046) when compared with uncoated MoM bearings at 13 months (Figures 1 and 2). A significantly increased level of Cr was measured in the MoM group when compared with the SLC-MoM group at 1 (p = 0.029), 3 (p = 0.024), 6 (p = 0.013), 12 (p = 0.019) and 13 (p = 0.010) months post operatively. A significantly increased Co level was measured in the MoM group when compared with SLC-MoM implants at 6 (p = 0.042), 12 (p = 0.011) and 13 (p = 0.011) months post operatively. Metallic debris was only seen within synovial tissue immediately adjacent to the implant bearing. A significantly lower ALVAL score was measured in the SLC-MoM group (3.88) when compared with MoM components (6.67) (p = 0.010). No significant difference was found when SLC-MoM and MoP (5.25) were compared. Pseudotumor, muscle atrophy and synovial thickening were not seen in the MRI scans.
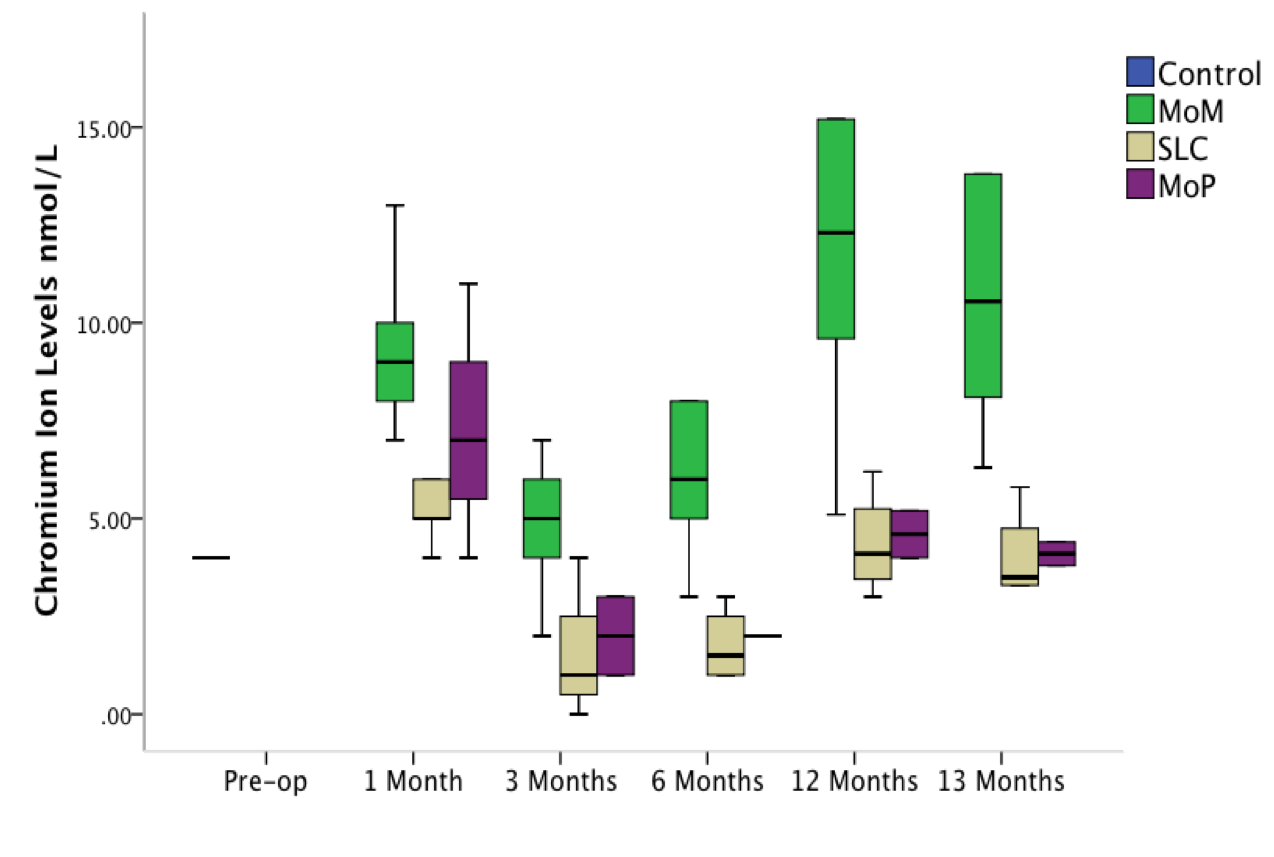
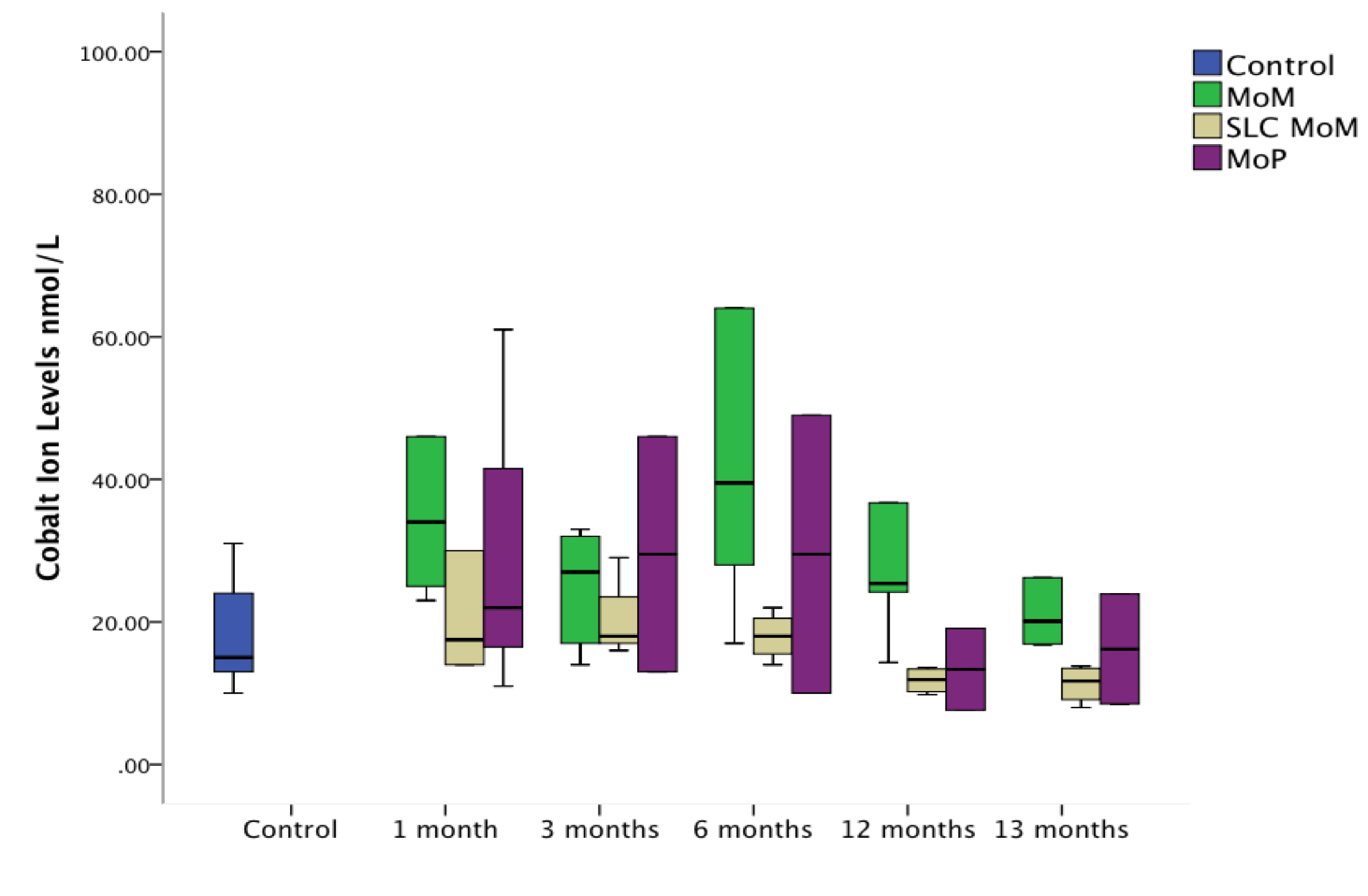
Discussion: This study successfully used a repeatable and reproducible accelerated total hip replacement ram model to study the effects of the wear and toxicity of released debris from different implant bearing materials in vivo. A significant reduction in Cr and Co ion release and a decrease in the incidence of ALVAL were achieved following the application of this 4th generation ceramic coating on articulating surfaces in metal-on-metal hip replacement surgery.