Introduction: Hyaluronic acid (HA) is an abundant glycosaminoglycan secreted by fibroblast-like synoviocytes (FLS) into the synovial fluid. UDP-glucose dehydrogenase (UDPGD) is an enzyme expressed in FLS and a reported rate-limiting enzyme in HA synthesis[1].
FLS and macrophages form the synovial membrane and modulate together the composition of the synovial fluid, including HA levels and bioactive factors[2],[3]. To develop novel approaches to stimulate endogenous HA release into the synovial fluid, more knowledge is needed on the effect of cytokines on UDPGD activity.
In this study, a novel in situ enzyme assay was developed to visualize UDPGD activity in primary FLS monolayer cultures, to test the hypothesis that anabolic, inflammatory and morphogenetic factors known to induce HA release [4] also up-regulate UDPGD activity. We also tested the role of cell-cell contact in UDPGD activity using a new plastic disc method to generate controlled FLS aggregates in vitro.
Methods: FLS cells were extracted from donated human synovial biopsies from consenting patients undergoing arthroscopic knee surgery (N=6) with mild synovitis and moderate knee pain, following approved protocols.
UDPGD in situ enzyme staining of in vitro cell cultures was adapted from Pitsillides [5], used an extra cryoprotection step prior to staining, and unfixed rabbit synovium cryosections as controls. Confluent FLS cultures were incubated 24 hours in serum-free medium with and without IL-1β, PDGF-bb, TGF-β1, or 10% serum. HA release was measured by ELISA per DNA content. Cells were stained for in situ UDPGD activity in 8-well CultureSlide chambers. To create controlled cell aggregates, a fixed number of cells were attached to a specific area by capillary force using a sterile plastic disc. UDPGD stain was quantified by densitometry. Differences were calculated using Statistica (v10).
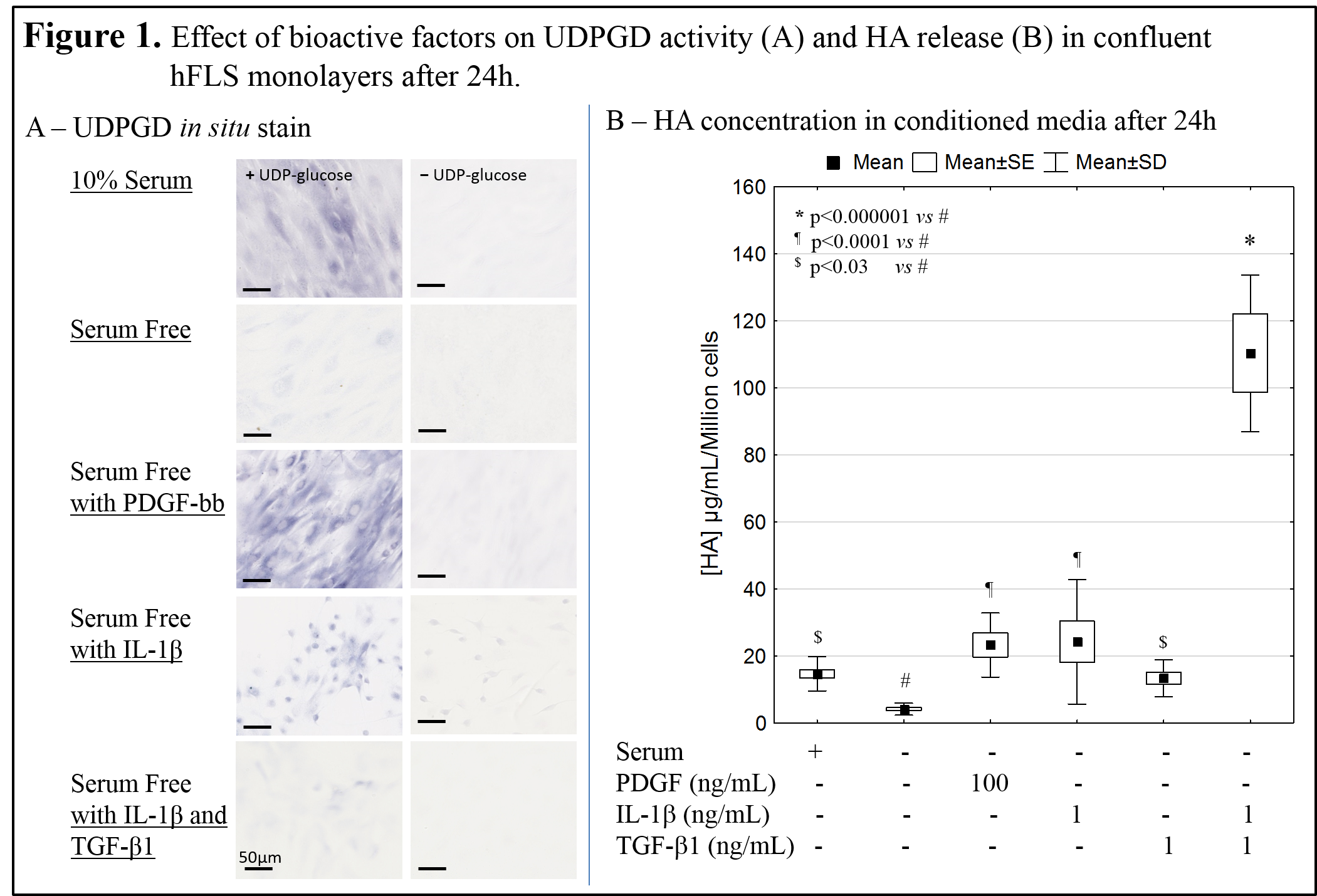
Results: Specific UDPGD staining in confluent FLS cultures was obtained by a freeze-thaw step in a buffer that maintained cell morphology and eliminated false-positive oxidase stain. UDPGD activity and HA secretion were very low in serum-free medium (Fig. 1). Despite donor-to-donor variation in UDPGD activity, staining results showed that UDPGD activity was intensified by serum, TGF-β1 and IL-1β and most strongly induced by PDGFbb (Fig. 1A). In vitro HA secretion in serum-free medium was increased 3-fold by serum and TGF-β1, and 6-fold by PDGFbb and IL-1β (Fig. 1B). The combination of TGF-β1 and IL-1β synergized to induce 30-fold greater HA release, with only minor UDPGD induction.
Correlation analyses did not reveal a linear correlation between HA release and UDPGD activity. Instead, results showed that cell aggregation was the strongest inducer of UDPGD, with or without serum or cytokines (Fig. 2A). Cell aggregation was insufficient to induce HA secretion in serum-free medium (Fig. 2B).
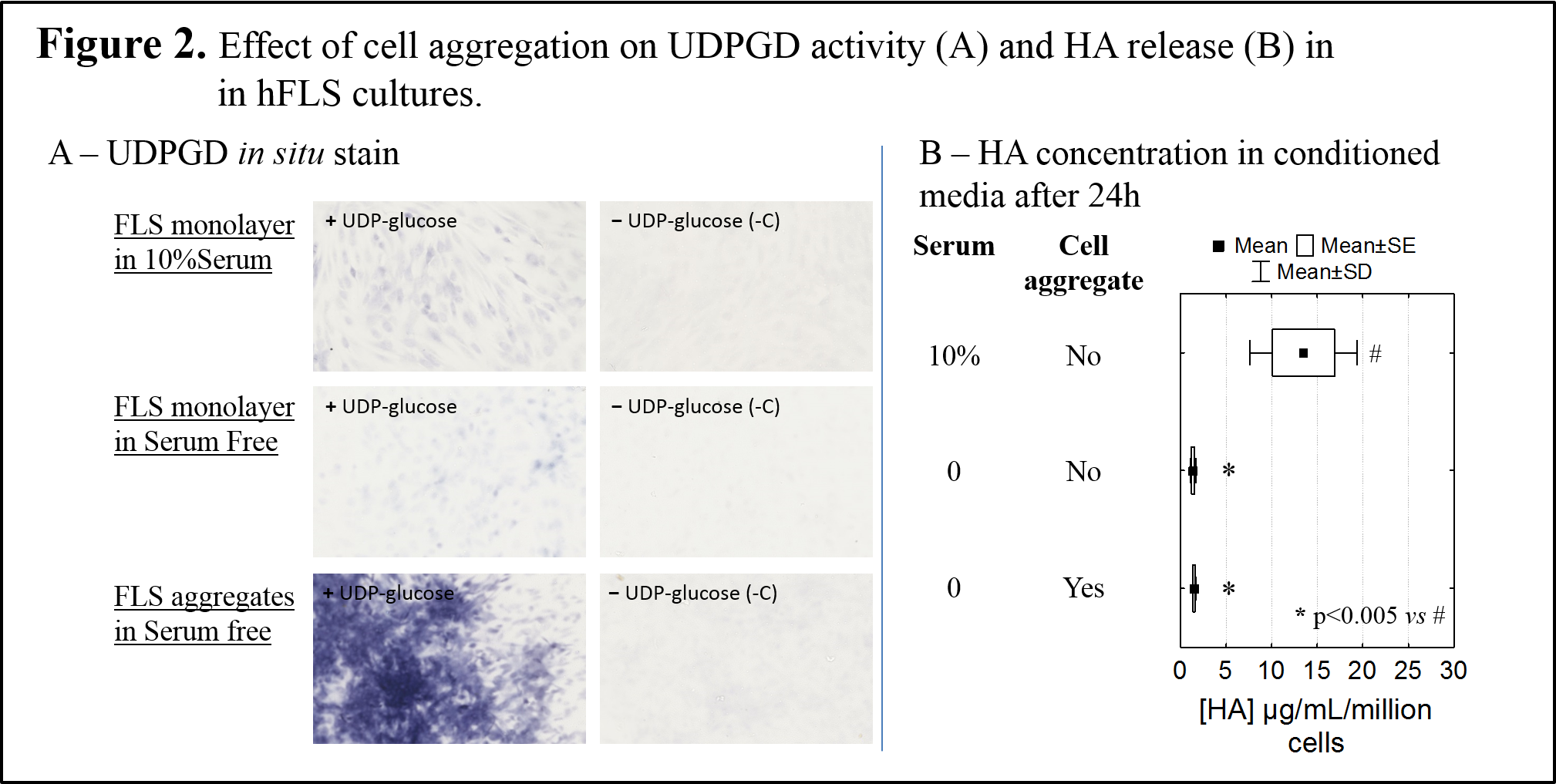
Discussion: Human FLS reproducibly up-regulated HA release in response to anabolic (PDGF-bb), catabolic (IL-1β) and morphogenetic (TFG-β1) factors. In these same conditions, we showed that UDPGD in situ enzyme activity is also induced, but not in direct proportion to the level of HA secreted in vitro. In addition, UDPGD activity was very low in the face of intense HA production that occurs under the synergic effect of catabolic and morphogenetic factors[4]. It was previously reported that UDPGD activity is suppressed in highly inflamed knee synovial tissue[5]. Data from this study suggest that the loss of UDPGD activity does not necessarily reflect loss of HA synthesis by FLS. The highest increase in UDPGD enzyme activity was observed in cell aggregates, which is consistent with the notion that cell-cell contact is a critical step prior to glycosaminoglycan deposition in the extracellular matrix[6].
Conclusion: UDPGD in situ activity was variably induced by inflammatory cytokines and growth factors previously shown to induce FLS to secrete HA in vitro. These same factors are also known to be released by macrophages under inflammatory conditions or after phagocytosis of certain biomaterials[7]. Intensification of UPDGD activity in cell aggregates may render cells competent to deposit glycosaminoglycan in the extracellular matrix.
Canadian Institutes of Health Research operating grant, funding source (CDH and RM); Natural Sciences and Engineering Council of Canada, funding source for a summer scholarship (AC); Mauri Zomar, for coordination of subject recruitment
References:
[1] Edwards JCW. The Nature and Origins of Synovium - Experimental Approaches to the Study of Synoviocyte Differentiation. Journal of Anatomy 1994; 184: 493-501.
[2] Kruger JP, Endres M, Neumann K, Haupl T, Erggelet C, Kaps C. Chondrogenic Differentiation of Human Subchondral Progenitor Cells Is Impaired by Rheumatoid Arthritis Synovial Fluid. Journal of Orthopaedic Research 2010; 28: 819-827.
[3] Benito MJ, Veale DJ, Fitzgerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Annals of the Rheumatic Diseases 2005; 64: 1263-1267.
[4] Recklies AD, White C, Melching L, Roughley PJ. Differential regulation and expression of hyaluronan synthases in human articular chondrocytes, synovial cells and osteosarcoma cells. Biochemical Journal 2001; 354: 17-24.
[5] Pitsillides AA, Blake SM. Uridine Diphosphoglucose Dehydrogenase-Activity in Synovial Lining Cells in the Experimental Antigen-Induced Model of Rheumatoid-Arthritis - an Indication of Synovial Lining Cell-Function. Annals of the Rheumatic Diseases 1992; 51: 992-995.
[6] Pitsillides AA, Archer CW, Prehm P, Bayliss MT, Edwards JCS. Alterations in Hyaluronan Synthesis during Developing Joint Cavitation. Journal of Histochemistry & Cytochemistry 1995; 43: 263-273.
[7] Fong D, Ariganello MB, Girard-Lauziere J, Hoemann CD. Biodegradable chitosan microparticles induce delayed STAT-1 activation and lead to distinct cytokine responses in differentially polarized human macrophages in vitro. Acta Biomaterialia 2015; 12: 183-194.