An average of $1.5 billion is spent trying to bring a new drug to the market, while a large portion of these drugs is withdrawn at the later stage of the development. Up to 20% of recent drug withdrawals were due to cardiac toxicity and proarrhythmic effects. 3D tissue constructs possess better physiological relevance and therefore provide more reliable toxicology responses comparing to conventional 2D tissue Thus, 3D tissue-specific toxicity screening with a human cardiac model should be used to weed out the unqualified drug candidates in a high throughput manner. The platform in this research enables batch production of 3D human cardiac tissues and can provide accurate force readouts as key reference for toxicity evaluation. The platform also incorporates array of electrodes to provide field stimulation of progressive frequency increase[1], which can significantly mature tissue to an infant-like state. The entire platform is made of polystyrene which provide a drug- inert environment to eliminate the drug absorption problem caused by polydimethylsiloxane (PDMS)[2].
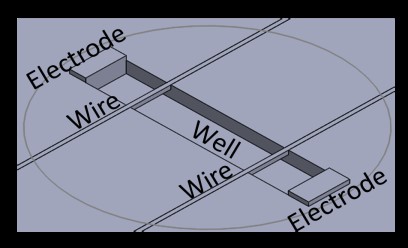
Hot embossing technique with polystyrene will generate micro-wells with dimension of 5mm by 1mm by 300mm, which will fit in a single well of a 96 well-plate. Every micro-well will have two auto-fluorescent and flexible wires made of poly(octamethylene maleate (anhydride) citrate) positioned at the edges of the well. After cell seeding, cardiac tissue will compact around wires and hang in the well. Gold printed electrodes will be located at both ends of the well and connected with electrical stimulators (Grass88X). Optical and fluorescence high speed camera will record shape changes of the wires over time during tissue contraction in the device. By tracking the deflection of the wire, beam deflection equation will be used to measure tissue generated contractive forces. Beating frequency, amplitude, contraction and relaxation times will be easily acquired as well. The accuracy of the device will be validated by comparing the calculated forces and the ones measured using a force transducer. This device can eliminate the common drawback of post deflection design, in which tissue often slips off the posts. The design will also prevent inconveniently measuring positions of tissue on posts, which is a crucial parameter in force calculation.
To evaluate drug induced toxicity towards cardiac tissue, drugs with known effects (e.g.verapamil, nifedipine, norepinephrine, isoproterenol, lidocaine, caffeine, E-4301, etc.) are tested to confirm ECT responds like human adult cardiac tissue. Then, a group of drugs approved but withdrawn from the market (cisparide, terfenadine, rosiglitazone) are tested to show the sensitivity of the device. Negative control will be ketaconazole with no known cardiotoxicity.
References:
[1] Nunes, S.S., et al., Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods, 2013. 10(8): p. 781-7.
[2] Toepke, M.W. and D.J. Beebe, PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip, 2006. 6(12): p. 1484-6.