Introduction: In the past two decades, vertebroplasty has been considered as a safe, efficient and common surgical procedure that plays a main role in the treatment of vertebral compression fractures. This technique has been applied in pain reduction and for preventing further loss of vertebral body height in osteoporosis[1]. Calcium sulphate cements (CSC) are nontoxic, osteoconductive, bioresorbable and have been applied in clinical practice for over 100 years. However, their degradation rate is faster than the formation of new bone, which has limited their application[2],[3]. This study aimed to overcome the drawbacks of calcium sulphate by developing an injectable material containing three phases.
Materials and Methods: The injectable cement formulation included: calcium sulphate hemihydrate (CSH-type III), a radiopaque glass ceramic and mesoporous bioactive glass particles. The glass-ceramic particles (mol. composition: SiO2 =57%, CaO=30%, Na2O=6% and ZrO2=7%) were prepared by melting, casting and ceramisation treatment. Mesoporous glass particles were synthesized by spray-drying technique using a water-based synthesis. In order to obtain a cement, the three phases were combined with an optimized liquid to powder ratio, which ensured that the paste remained injectable and completely set in proper time. The new cement was studied in vitro both in simulated body fluid (SBF) and by culturing rat bone marrow stromal cells (BMSC). The behaviour of the calcium sulphate-based composite cement (setting time, injectability, compressive strength) was evaluated with respect to type III calcium sulphate and Cerament® (as a commercial reference).
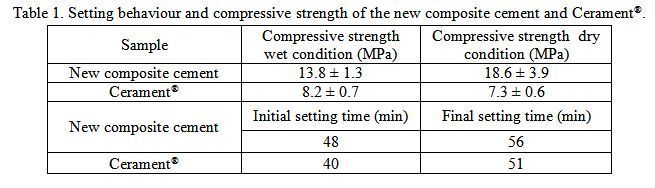
Results: It was found that the new composite cement possessed slightly higher initial (48 min) and final (56 min) setting times, respectively (table 1). Moreover, the injectable cement showed a better compressive strength in both wet (13.8MPa) and dry (18.6MPa) conditions with respect to the commercial reference (table 1). The new cement samples were biodegradable and significantly improved bioactivity during the first week in vitro and this may enhance new bone formation in vivo (figure 1). The in vitro cell culture results proved that the new cement are cytocompatible, and might enhance osteogenic differentiations of the BMSC by increasing alkaline phosphatase activity.
The new formulation of the injectable cement for vertebroplasty showed good setting times and compressive strength, and in vitro osteogenic properties which are comparable or better than Cerament®. This composite cement might has significant clinical advantages compared to the traditional CSH.
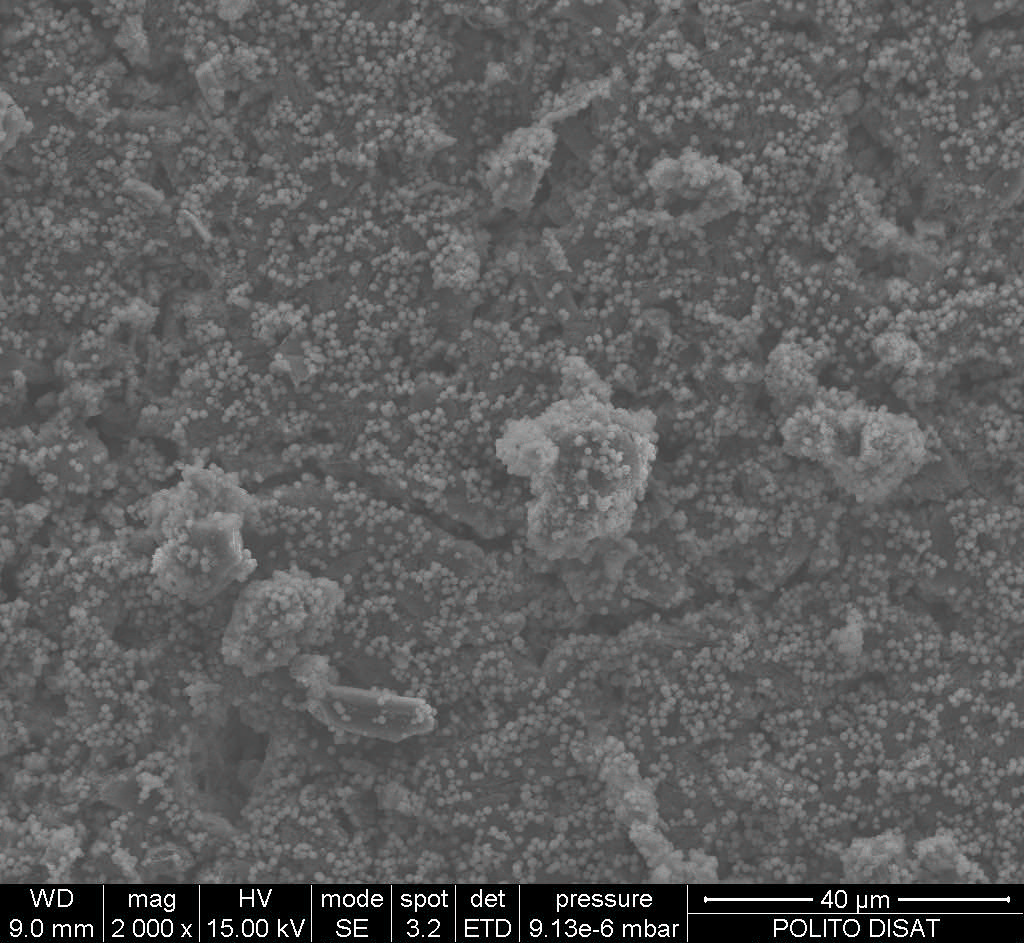
Fig. 1. FESEM micrograph of the composite cement after 24 hr of SBF soaking showing with hydroxyapatite crystals.
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n°[280575]-Restoration
References:
[1] Todd McCall, Chad Cole, Andrew Dailey, “Vertebroplasty and kyphoplasty: a comparative review of efficacy and adverse events”, Curr Rev Musculoskelet Med (2008) 1:17–23 DOI 10.1007/s12178-007-9013-0.
[2] Andrew Perry, Andrew Mahar, Jennifer Massie, Noemi Arrieta, Steven Garfin, Choll Kim, “Biomechanical evaluation of kyphoplasty with calcium sulfate cement in a cadaveric osteoporotic vertebral compression fracture model”, The Spine Journal 5 (2005) 489–493.
[3] Gangfeng Hu, Luwei Xiao, Hong Fu, Dawei Bi, Haitao Ma, Peijian Tong, “Study on injectable and degradable cement of calcium sulphate and calcium phosphate for bone repair”, J Mater Sci: Mater Med (2010) 21:627–634 DOI 10.1007/s10856-009-3885-z.