Introduction: For their high strength and wear properties, CoCr alloys find extensive applications in orthopaedic reconstructive surgery[1],[2]. However, their biomechanical fixation and capacity to support osseointegration are considered inferior to titanium (typically Ti6Al4V) alloys[3],[4]. In contrast to machined implants having a solid macro-shape and little possibility for bone ingrowth, electron beam melting (EBM) technology provides possibilities to build complex geometries in a predetermined controlled design (e.g., constructs with controlled open-pore architecture). This work evaluates bone growth and osseointegration of 3D printed porous CoCr and Ti6Al4V constructs.
Materials and Methods: CoCr and Ti6Al4V constructs were produced from atomised alloy powders in an Arcam EBM system. Five adult sheep received, bilaterally, either two CoCr or Ti6Al4V implants in each femoral epiphysis (n=10), which were retrieved en bloc after six months of healing. Histomorphometry was performed using toluidine blue stained ground-sections prepared from each specimen. The remaining material was used for Raman spectroscopy and direct visualisation by scanning electron microscopy (SEM) of the osteocyte lacuno-canalicular network by resin-cast etching. The Wilcoxon signed-rank test was used for statistical analysis of the histomorphometric data; p < 0.05 was considered statistically significant.
Results and Discussion: CoCr and Ti6Al4V constructs of similar open-pore architecture (Fig. 1a) were well integrated, with large amounts of organised, mineralised lamellar bone within and around the porous network (Fig. 1b). No signs of adverse tissue response, e.g., fibrous encapsulation, were evident. Both inside and outside the porous network, the presence of resorption areas containing a few osteoclasts, simultaneous with osteoid deposition by osteoblast seams indicated bone remodelling. While the bone area (BA) for both materials was comparable, both inside and outside the porous network, the bone-implant contact (BIC) was lower for CoCr (Fig. 1c). However, as no biomechanical test could be performed, the significance of lower BIC for such complex implant geometries is yet to be determined. Using Raman spectroscopy, the interfacial composition (5-10 µm from the implant surface) in areas of direct bone-implant contact was comparable for CoCr and Ti6Al4V. The mineral crystallinity, the mineral-to-matrix ratio, and the carbonate-to-phosphate ratio were similar for the two materials (Fig. 1d). Osteocytes were seen to make direct contact with both alloy surfaces (Fig. 1e).
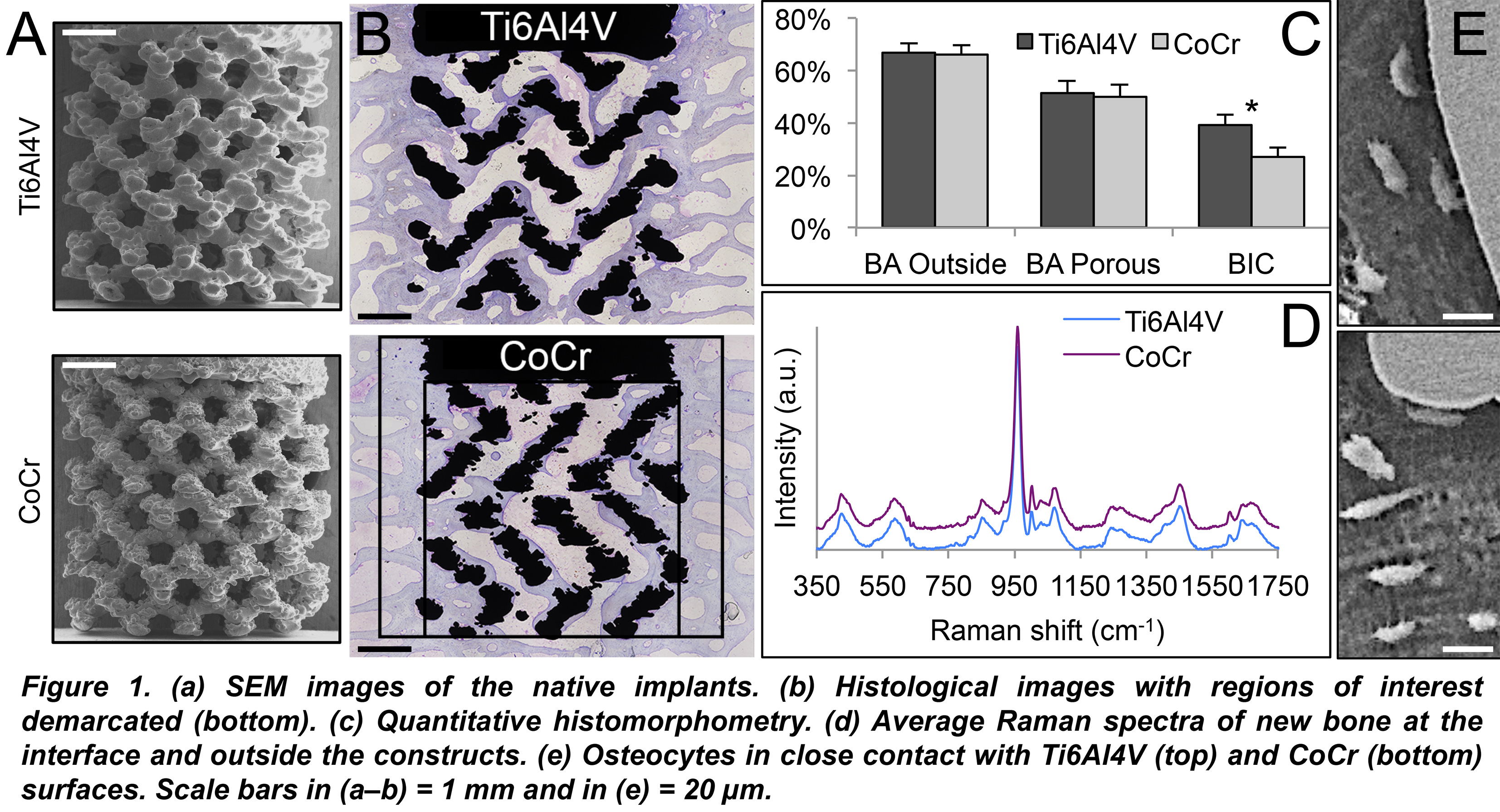
Conclusions: Within the limitations of the present work, 3D printed CoCr implants display biocompatibility, potential for bone ingrowth and osseointegration to the same extent as Ti6Al4V with obvious advantages for high load-bearing applications. The formed bone undergoes continuous renewal, irrespective of the location, i.e., inside or around the interconnected porous network. The lower BIC for CoCr, however, may have implications for biomechanical fixation.
This study was supported by the Swedish Research Council (grant K2015-52X-09495-28-4), the BIOMATCELL VINN Excellence Center of Biomaterials and Cell Therapy, the Region Västra Götaland, an ALF/LUA grant, the IngaBritt and Arne Lundberg Foundation, the Dr. Felix Neubergh Foundation, Promobilia, the Hjalmar Svensson Foundation, the Adlerbertska Foundation, and the Swedish Government Strategic Funding of the Materials Science Area of Advance.
References:
[1] Saldivar-Garcia AJ, Lopez HF. J Biomed Mater Res A. 2005;74:269.
[2] Chiba A, Kumagai K, Nomura N, Miyakawa S. Acta Mater. 2007;55:1309.
[3] Jinno T, Goldberg VM, Davy D, Stevenson S. J Biomed Mater Res. 1998;42:20.
[4] Jakobsen SS, Baas J, Jakobsen T, Soballe K. J Biomed Mater Res A. 2010;94:180.