Introduction: The major frontier to establishing tissue-scale biomimetic 3D constructs remains the ability to synergistically incorporate a vascular-like perfusion system capable of supporting metabolic demands of embedded cells. Despite recent advances, fabrication of vascularized cell scaffolds remains an engineering challenge. Here we describe using sacrificial, melt-spun polymeric fibers to generate an interconnected channel network in gelatin hydrogels with dimensional and architectural complexity difficult to achieve with other top-down fabrication approaches. Towards the goal of achieving a fully cellularized microvascular lumen within a hydrogel construct, this work details progress in (1) establishing robust fluidic interface mechanism for perfusion, (2) characterization of microchannel network, and (3) optimization of cell seeding and confluent growth within microchannels.
Materials and Methods: To optimize fluidic interface of free-standing hydrogel scaffolds, we evaluated the performance of both adhesive (gelatin, fibrin, and cyanoacrylate based compounds) and non-adhesive (pressure fit barb or silicon tubing) connection modalities in test devices of varying stiffness comprised from gelatin (7%, 10%, and 12% w/v), alginate (3% w/v), and PDMS. Burst pressure and tensile strength measurements were obtained using fluidic pressure sensor (Honeywell) and Instron 5944 (extension rate of 2 mm/min), respectively (Fig. 1). Cytocompatibility was assessed by confocal (LSM710, Zeiss) imaging live/dead staining of RFP-expressing HUVECS (Angio-Proteomie) cultured in perfused channels for 72 hours. Microchannel devices were fabricated from melt-spun Joncryl 678 (BASF) fibers embedded in porcine gelatin (10% w/v, Sigma) crosslinked with 1% (w/v) microbial transglutaminase (mTG, Modernist Pantry). pH-triggered dissolution of Joncryl fibers was achieved by immersion in 30 mM ammonia baths for 24 hours. Confocal images of microchannels illuminated with 0.04 μm fluorescent microspheres were analysed to characterize network architecture[1]. Device macrochannels were seeded with RFP-HUVECs under static conditions, followed by fluidic introduction of additional cells into microchannels. Cell scaffolds were maintained under continual perfusion (100 μl/min) and viability, confluency, and cell-cell adhesion were monitored by confocal imaging of live/dead indicators, PECAM/VE-cadherin expression, and dextran diffusion.
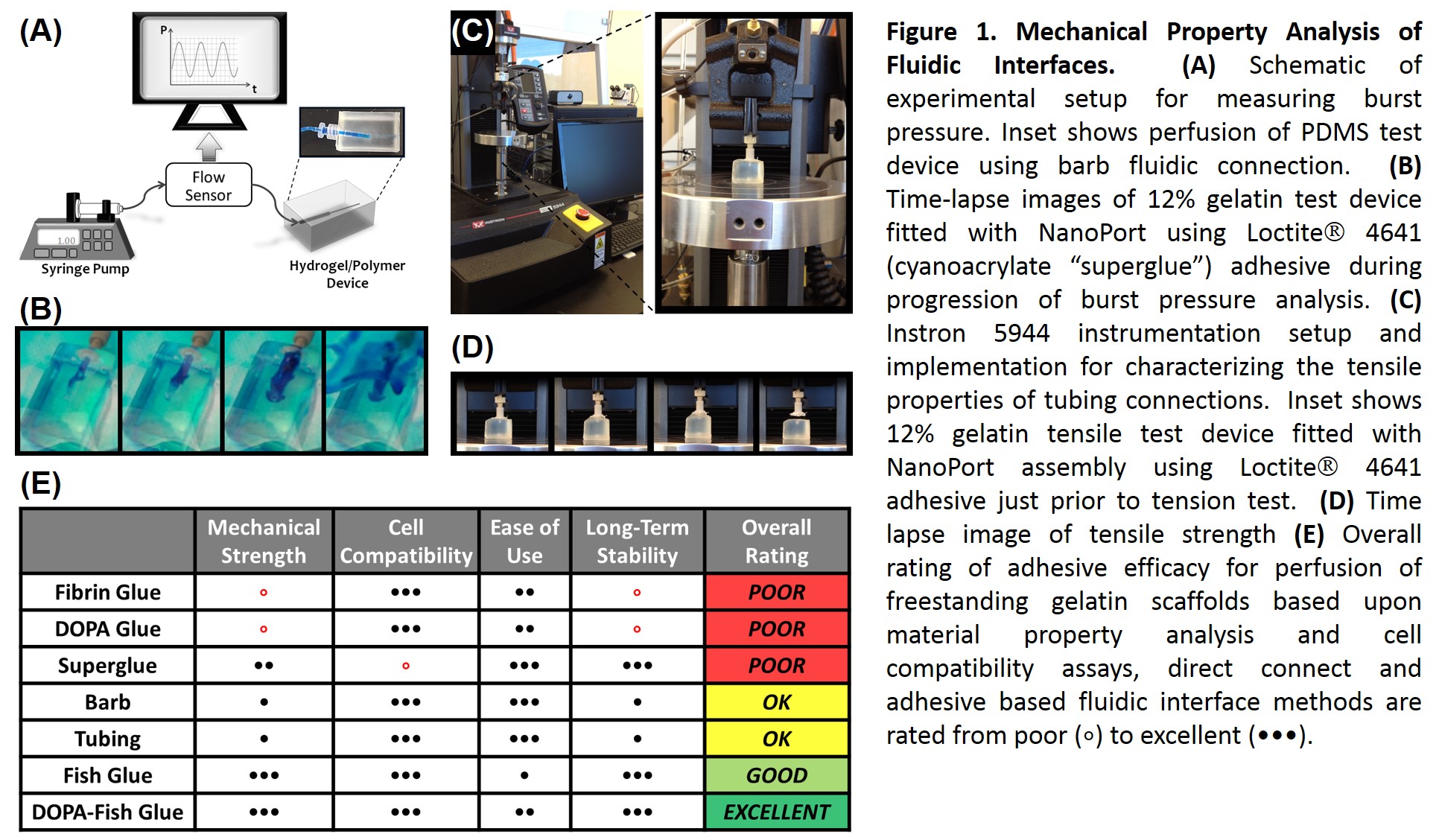
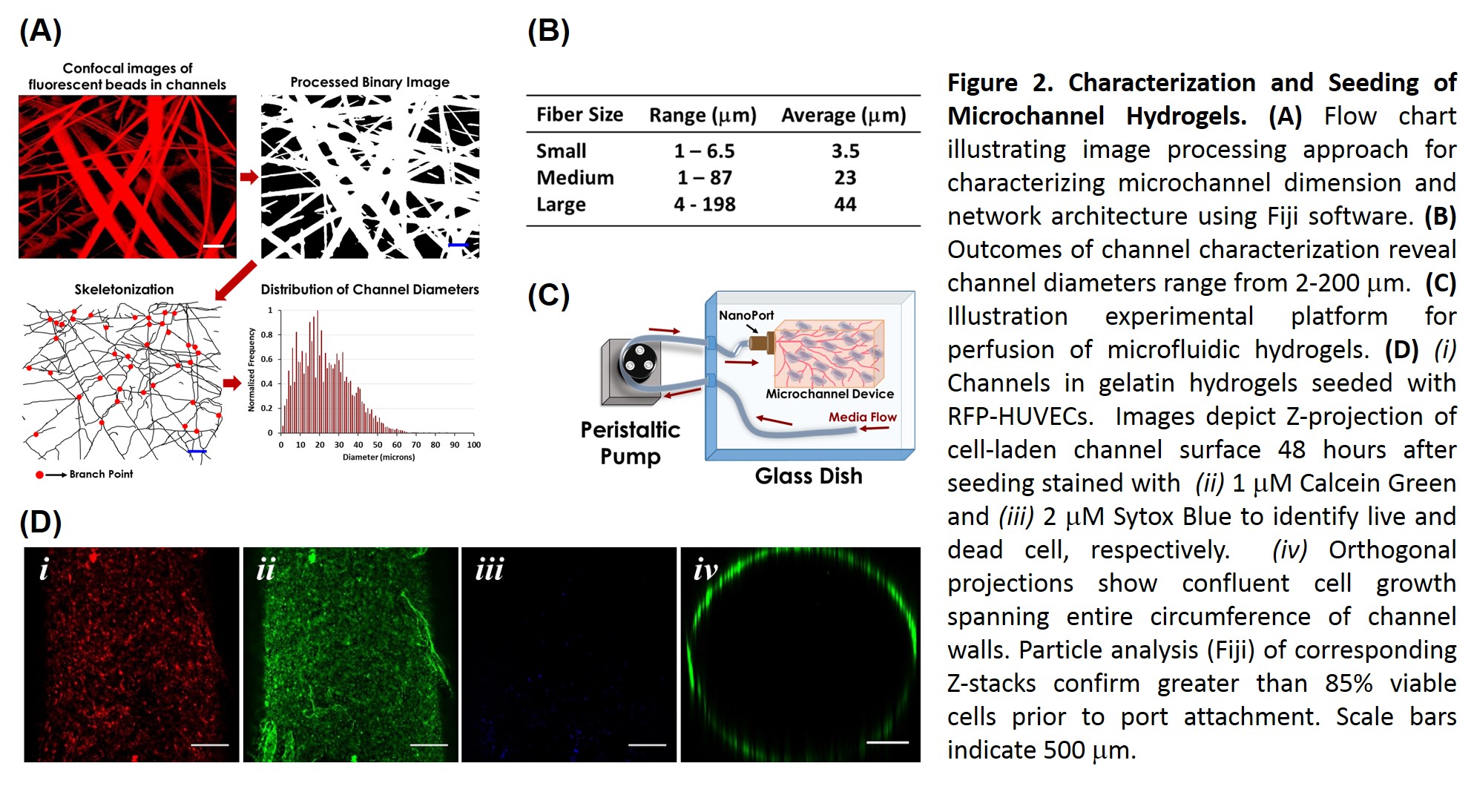
Results and Discussion: Efficacy of fluidic connection modalities was highly dependent upon substrate composition. For gelatin hydrogels, superglue was highly effective, but was highly cytotoxic. DOPA glue, a novel combination of L-DOPA modified porcine gelatin[2] and mTG crosslinked cold-water fish gelatin, proved to be both a robust adhesive and cell compatible. Imaging analysis of channel architecture revealed channel diameters ranging from 2-200 μm, corresponding to capillary and arteriole-sized lumens, with inter-channel spacing of less than 200 μm. Confluent seeding of device macrochannels was achieved within 24 hours. Seeding of dense microchannel network proved more challenging, but optimizing cell density, loading techniques, and perfusion media yielded cellularized regions viable for over 10 days (Fig. 2). Efforts to optimize the speed and efficiency cell seeding throughout the ~19 cm3 scaffold volume are underway.
Conclusions: This work validates the use of sacrificial fibers for generating immediately perfusable hydrogels containing physiologically inspired, cell-laden microchannel lumen. Utilizing a top-down fabrication approach, allows for rapid establishment of, tissue-scale 3D scaffold assemblies, with promising implications for advances in tissue engineering applications. Future efforts will focus on optimizing sacrificial biomaterial and dissolution methods to better facilitate incorporation of multicellular components.
NIH 4R00EB013630 (NIBIB)
References:
[1] Bellan LM, Pearsall M, Cropek DM, Langer R. A 3D Interconnected Microchannel Network Formed in Gelatin by Sacrificial Shellac Microfibers. Adv Mater 2012;24:5187–91. doi:10.1002/adma.201200810.
[2] Chan Choi Y, Choi JS, Jung YJ, Cho YW. Human gelatin tissue-adhesive hydrogels prepared by enzyme-mediated biosynthesis of DOPA and Fe3+ ion crosslinking. J Mater Chem B 2014;2:201–9. doi:10.1039/C3TB20696C.