Introduction: Non-union bone defects lead to patient suffering and a substantial expenditure of healthcare monies[1]. Clinical approaches, such as bone autograft, are somewhat successful but have limitations[1]. Artificial bone graft substitute materials potentially offer an inexpensive solution that avoids the disadvantages of autografts such as the need for an additional surgical procedure and a finite supply of bone. However, current bone graft substitutes fail to mimic bone’s mechanical, chemical and biological properties, preventing their widespread clinical application. Here, we design and test a material with similar mechanical properties as bone, has phosphate groups that mimic bone apatite, and can be functionalized to promote osteogenesis.
Materials and Methods: Graphene oxide (GO) was prepared using a modified Hummers’ method. Phosphate-functionalized GO (P-GO) was prepared using a modified Arbuzov reaction with Li, Na, Mg, K, and Ca counter ions for Br.[2] Fluorescein was conjugated to GO (F-GO) via an esterification reaction. After purification, the materials were characterized with Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and absorbance spectroscopy. Suspensions were prepared at 1 mg/mL in water and exposed to RAW 264.7 macrophages and NIH-3T3 fibroblasts. Cellular proliferation was assessed using cell enumeration; metabolism was assessed using Calcein AM; and late apoptosis and necrosis were assessed via propidium iodide (PI) exclusion. Confocal imaging was performed using a Zeiss LSM 510 Meta DuoScan spectral confocal microscope.
Results and Discussion: Absorbance spectroscopy, FTIR, and TGA confirmed the phosphate functionalization of GO (Fig. 1).
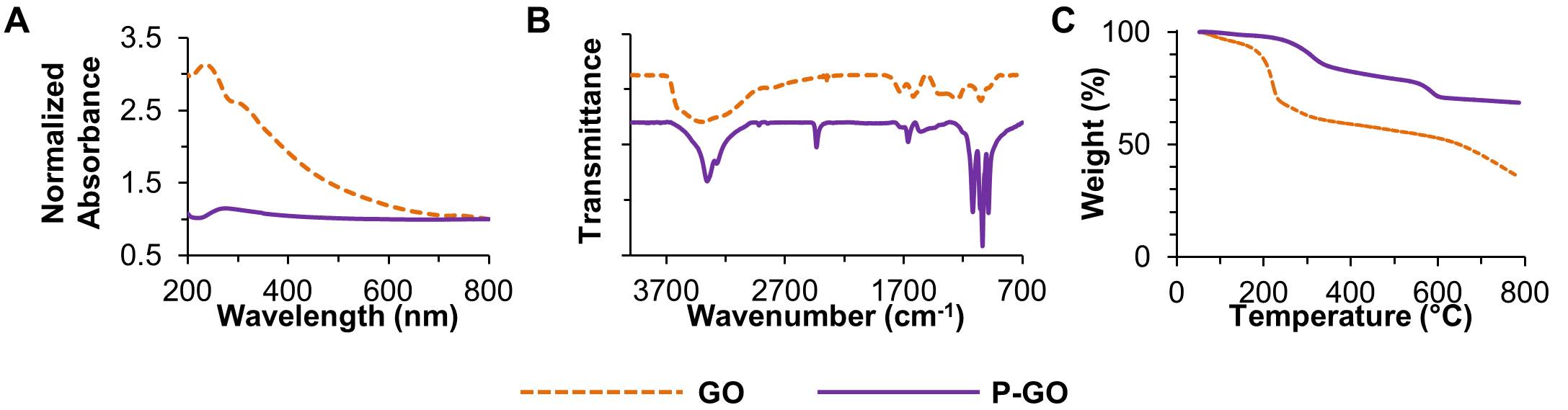
Figure 1: (A) Absorbance spectroscopy, (B) FTIR, and (C) TGA of GO and P-GO.
Cellular exposure to suspensions of P-GO diluted in cell culture media did not alter cellular proliferation, metabolism, or apoptosis, regardless of counter ion (Fig. 2).
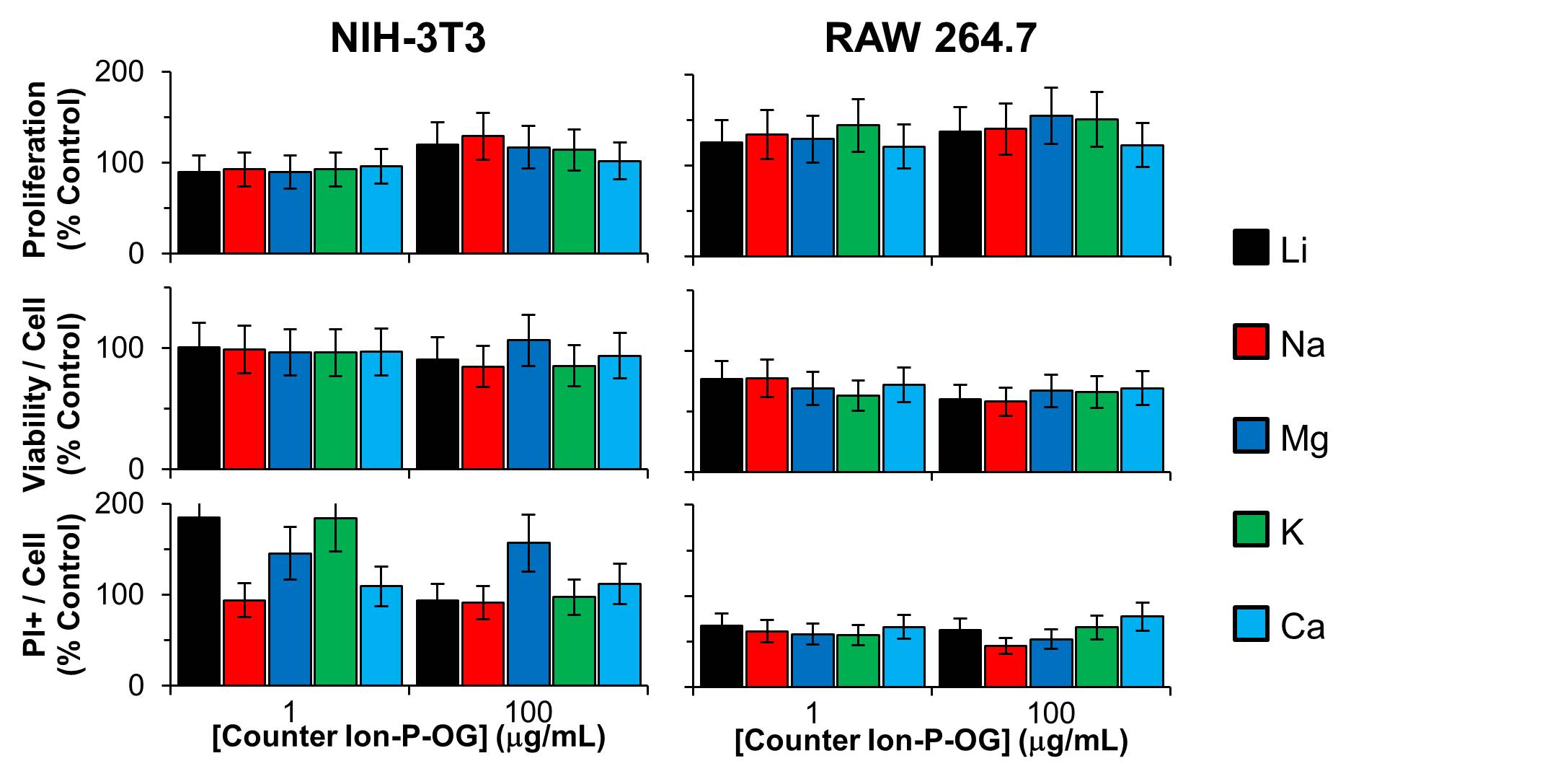
Figure 2: Dose-dependent in vitro cytocompatibility for the indicated counter ion used in the Arbuzov reaction. Error bars are standard deviation, and the data are normalized to control.
Confocal imaging revealed that GO and, with higher sensitivity, F-GO were associated with cells but did not co-register with nuclei and did not substantially alter nuclear shape (Fig. 3).
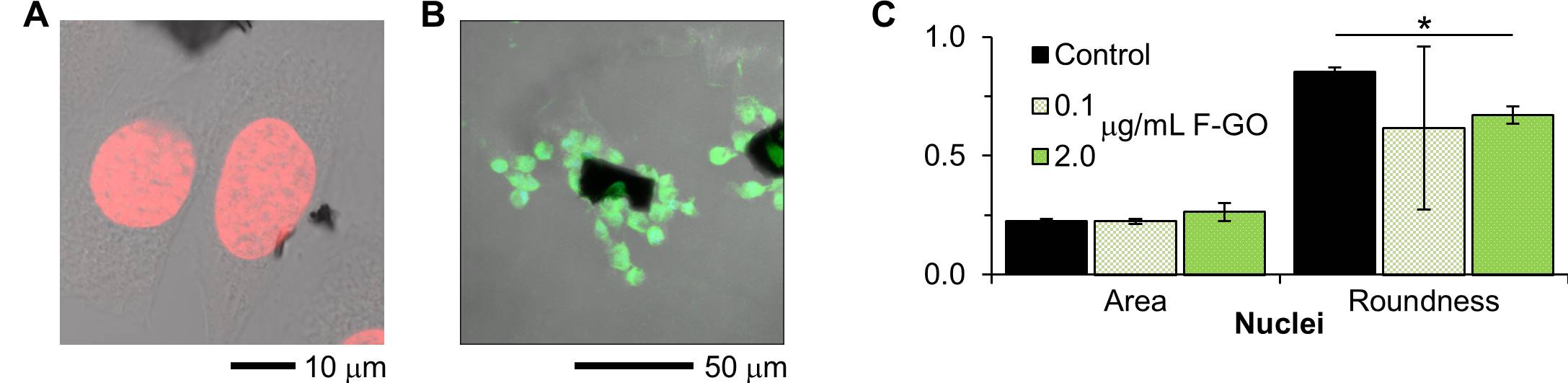
Figure 3: (A) Confocal Z-slice of NIH-3T3 fibroblasts exposed to GO. The black regions are GO, the nuclei are red, and cellular morphology is imaged with DIC. (B) Confocal compressed Z-stack of RAW 264.7 macrophages exposed to F-GO. The cells are imaged with DIC, and green signal arises from fluorescein. (C) Quantification of nuclei shape from confocal images. The slight reduction in roundness for F-GO is probably related to a reduction in cell density.
Conclusion: P-GO was synthesized and is cytocompatible. Previous reports show that P-GO processed into pellets has similar mechanical properties to that of bone[2] and GO is moderately compatible in vivo[3]. Since the chemical and mechanical properties mimic that of bone and the particulates which would be part of the materials’ degradation are cytocompatible, these results indicate that this material holds promise as a bone graft substitute material. These materials may be further functionalized with osteogenic moieties or collagen for crosslinking to serve as a cytocompatible, bioactive bone graft substitute.
References:
[1] The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Costs. 3rd ed.; United States Bone and Joint Initiative: 2011.
[2] Goods, J. B.; Sydlik, S. A.; Walish, J. J.; Swager, T. M., Phosphate Functionalized Graphene with Tunable Mechanical Properties. Advanced Materials 2014, 26 (5), 718-723.
[3] Sydlik, S. A.; Jhunjhunwala, S.; Webber, M. J.; Anderson, D. G.; Langer, R., In Vivo Compatibility of Graphene Oxide with Differing Oxidation States. ACS Nano 2015, 9 (4), 3866-3874.