Introduction: The most dramatic complication of intraocular lenses (IOLs) implantation is endophthalmitis, an infection caused by bacteria that may occur in the post-surgical period. It may cause severe inflammation with risk of corneal opacification and even eye loss. The use drug-loaded IOLs to prevent this problem has been regarded with great interest. Drug-loaded soft contact lenses (SCLs) also seem to constitute a promising vehicle for drug delivery that can be used in the treatment of a vast number of pathologies of the anterior part of the eye. However, these devices still do not exist in the market.
In its development, it is essential to ensure that all the established specifications are satisfied, including regulations relative to microbial contamination. Although well-established terminal sterilization methods are available, concerns have raised regarding the undesirable effects that these techniques may have on the hydrogels. More, the effects of sterilization procedures on drug-loaded hydrogels, including changes both on the intrinsic properties of the materials and on the drug release behavior, are unknown. Another important issue is the effect of sterilization on the activity of the loaded drugs. This work aims to contribute for the clarification of the effects of different methods of sterilization on drug-loaded SCLs and IOLs.
Materials and Methods: Solutions of drugs (levofloxacin, moxifloxacin, diclofenac and ketorolac), two commercial SCLs (Acuvue®Oasys® and 1-Day Acuvue®TruEye®), one material under study for SCLs (POLY(METH)ACRYLIC HYDROGEL ) and one for IOLs (HYDROPHILIC ACRYLATE WITH 26% WATER UPTAKE and) were sterilized by different methods (steam and pressure: 60 min at 121⁰C and 1 bar; and γ-radiation: 5, 15 and 25 kGy). Drug solutions prepared in NaCl 0,9% were sterilized by steam and with γ-radiation. The drugs were also irradiated in the form of powder and in NaCl solution containing 5% of mannitol. The lenses materials were sterilized in NaCl solution. The drug concentrations in the solutions were quantified before and after sterilization by high-performance liquid chromatography (HPLC). The ocular lenses materials were characterized with respect to transparency (UV-Vis spectroscopy), wettability (captive bubble method) and swelling behavior at 36⁰C.
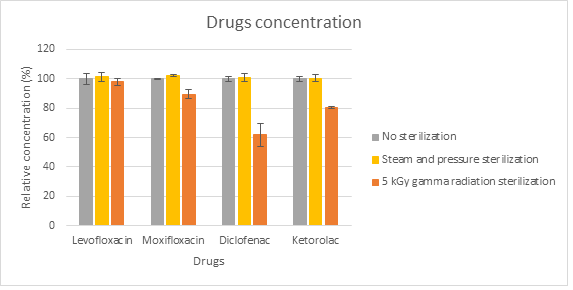
Results and Discussion: The drugs sterilized with steam did not suffer degradation. The γ-radiation led to different results depending on the form of presentation of the drugs: powders were not degraded; solutions with NaCl, with and without mannitol, did not present significant differences, leading to the conclusion that mannitol at 5% does not prevent the degradation of the tested drugs. Concerning the radiation doses, generally 15 kGy and 25 kGy degraded all drugs, so these doses were abandoned. At 5 kGy, the antibiotics did not present significant degradation in both solutions; however, the anti-inflammatories suffered degradation at this dose, being diclofenac the most affected.
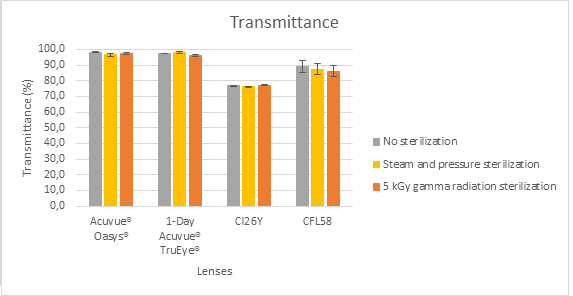
For the lenses materials, steam sterilization did not affect significantly their transmittance and swelling behaviour, but the wettability of both commercial SCLs slightly decreased while that of HYDROPHILIC ACRYLATE WITH 26% WATER UPTAKE slightly increased. The γ-radiation sterilization led to a small decrease in transmittance of POLY(METH)ACRYLIC HYDROGEL and enhanced the swelling behaviour only for Acuvue®Oasys®.
Conclusion: Steam and γ-radiation at 5 kGy seem to be promising methods for terminal sterilization of drugs and lenses. Next stage of this work is to study the drug release behavior before and after sterilization.
Fundação para a Ciência e Tecnologia (FCT), Project SurfLenses - MERA.NET/0005/2012.; Fundação para a Ciência e Tecnologia (FCT), Project UID/QUI/00100/2013
References:
[1] Terryn H, Maquille a, Houeelevin C, Tilquin B.. Int. J. Pharm. 2007;343(1-2):4-11.
[2] Maquille A, Jiwan JLH, Tilquin B.. Int. J. Pharm. 2008;349(1-2):74-82.